blackbody
advertisement

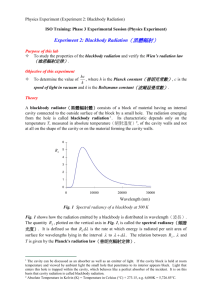
Black Body radiation Hot filament glows. Classical physics cant explain the observed wavelength distribution of EM radiation from such a hot object. This problem is historically the problem that leads to the rise of quantum physics during the turn of 20th century Need for Quantum Physics Problems remained from classical mechanics that relativity didn’t explain Attempts to apply the laws of classical physics to explain the behavior of matter on the atomic scale were consistently unsuccessful Problems included: blackbody radiation The electromagnetic radiation emitted by a heated object photoelectric effect Emission of electrons by an illuminated metal Quantum Mechanics Revolution Between 1900 and 1930, another revolution took place in physics A new theory called quantum mechanics was successful in explaining the behavior of particles of microscopic size The first explanation using quantum mechanics was introduced by Max Planck Many other physicists were involved in other subsequent developments Blackbody Radiation An object at any temperature is known to emit thermal radiation Characteristics depend on the temperature and surface properties The thermal radiation consists of a continuous distribution of wavelengths from all portions of the em spectrum Blackbody Radiation, cont. At room temperature, the wavelengths of the thermal radiation are mainly in the infrared region As the surface temperature increases, the wavelength changes It will glow red and eventually white The basic problem was in understanding the observed distribution in the radiation emitted by a black body Classical physics didn’t adequately describe the observed distribution Blackbody Radiation, final A black body is an ideal system that absorbs all radiation incident on it The electromagnetic radiation emitted by a black body is called blackbody radiation Blackbody Approximation A good approximation of a black body is a small hole leading to the inside of a hollow object The hole acts as a perfect absorber The nature of the radiation leaving the cavity through the hole depends only on the temperature of the cavity Blackbody Experiment Results The total power of the emitted radiation increases with temperature Stefan’s law (from Chapter 20): P = sAeT4 The peak of the wavelength distribution shifts to shorter wavelengths as the temperature increases Wien’s displacement law lmaxT = 2.898 x 10-3 m.K Real life blackbody A close approximation of blackbody radiator The colour of the light emitted from the charcoal depends only upon the temperature example This figure shows two stars in the constellation Orion. Betelgeuse appears to glow red, while Rigel looks blue in color. Which star has a higher surface temperature? (a) Betelgeuse (b) Rigel (c) They both have the same surface temperature. (d) Impossible to determine. Stefan’s Law – Details P = sAeT4 P is the power s is the Stefan-Boltzmann constant s = 5.670 x 10-8 W / m2 . K4 Stefan’s law can be written in terms of intensity I = P/A = sT4 For a blackbody, where e = 1 Wien’s Displacement Law lmaxT = 2.898 x 10-3 m.K lmax is the wavelength at which the curve peaks T is the absolute temperature The wavelength is inversely proportional to the absolute temperature As the temperature increases, the peak is “displaced” to shorter wavelengths Intensity of Blackbody Radiation, Summary The intensity increases with increasing temperature The amount of radiation emitted increases with increasing temperature The area under the curve The peak wavelength decreases with increasing temperature Exmple Find the peak wavelength of the blackbody radiation emited by (A) the Sun (2000 K) (B) the tungsten of a lightbulb at 3000 K Solutions (A) the sun (2000 K) By Wein’s displacement law, 3 2.898 10 mK lmax 2000K 1.4 m (infrared) (B) the tungsten of a lightbulb at 3000 K lmax 3 2.898 10 mK 0.5 m Yellow-green 5800K Rayleigh-Jeans Law An early classical attempt to explain blackbody radiation was the RayleighJeans law 2πck BT I λ,T λ4 At long wavelengths, the law matched experimental results fairly well Rayleigh-Jeans Law, cont. At short wavelengths, there was a major disagreement between the Rayleigh-Jeans law and experiment This mismatch became known as the ultraviolet catastrophe You would have infinite energy as the wavelength approaches zero Max Planck Introduced the concept of “quantum of action” In 1918 he was awarded the Nobel Prize for the discovery of the quantized nature of energy Planck’s Theory of Blackbody Radiation In 1900 Planck developed a theory of blackbody radiation that leads to an equation for the intensity of the radiation This equation is in complete agreement with experimental observations He assumed the cavity radiation came from atomic oscillations in the cavity walls Planck made two assumptions about the nature of the oscillators in the cavity walls Planck’s Assumption, 1 The energy of an oscillator can have only certain discrete values En En = nhƒ n is a positive integer called the quantum number h is Planck’s constant ƒ is the frequency of oscillation This says the energy is quantized Each discrete energy value corresponds to a different quantum state Planck’s Assumption, 2 The oscillators emit or absorb energy when making a transition from one quantum state to another The entire energy difference between the initial and final states in the transition is emitted or absorbed as a single quantum of radiation An oscillator emits or absorbs energy only when it changes quantum states Energy-Level Diagram An energy-level diagram shows the quantized energy levels and allowed transitions Energy is on the vertical axis Horizontal lines represent the allowed energy levels The double-headed arrows indicate allowed transitions More About Planck’s Model The average energy of a wave is the average energy difference between levels of the oscillator, weighted according to the probability of the wave being emitted This weighting is described by the Boltzmann distribution law and gives the probability of a state being occupied as being proportional to e E kBT where E is the energy of the state Planck’s Model, Graphs Planck’s Wavelength Distribution Function Planck generated a theoretical expression for the wavelength distribution 2πhc 2 I λ,T 5 hc λk T B λ e 1 h = 6.626 x 10-34 J.s h is a fundamental constant of nature Planck’s Wavelength Distribution Function, cont. At long wavelengths, Planck’s equation reduces to the Rayleigh-Jeans expression At short wavelengths, it predicts an exponential decrease in intensity with decreasing wavelength This is in agreement with experimental results Example: quantised oscillator vs classical oscillator A 2.0 kg block is attached to a massless spring that has a force constant k=25 N/m. The spring is stretched 0.40 m from its EB position and released. (A) Find the total energy of the system and the frequency of oscillation according to classical mechanics. Solution (A) Mechanical Energy, E = ½ kA2 = …= 2.0 J 1 k Frequency, f ... 0.56Hz 2 m (B) Assuming that the energy is quantised, find the quantum number n for the system oscillating with this amplitude. QUICK QUIZ 40.1 (For the end of section 40.1) In a certain experiment, you pass a current through a wire and measure the spectrum of light that is emitted from the glowing filament. For a current I1, you measure the wavelength of the highest intensity (also called lmax) to be l1. You then increase the voltage across the wire by a factor of 8 and the current increases by a factor of 2 to 2I1. The wavelength of the highest intensity will shift to a) 4l1, b) 2l1, c) 2l1, d) l1/ 2, e) l1/ 2, or f) l1/ 4. QUICK QUIZ 40.1 ANSWER (e). Assuming that the wire behaves like a blackbody, the wavelength with the highest intensity will be inversely proportional to the absolute temperature according to Wien’s displacement law, or lmax 1/T. In addition, the power radiated from the wire is proportional to the absolute temperature to the fourth power; from Stefan’s law, or P T4. Also, the power dissipated in the wire is given by P = IV. Combining these equations, one finds that, 1 1 1 1 1 lmax 1/ 4 . 1/ 4 1/ 4 T P ( IV ) (2 8) 2 QUICK QUIZ 40.2 The oscillators in a blackbody may oscillate a) at only certain frequencies, b) with only certain energies, c) at only certain frequencies and with only certain energies, d) with only certain energies for a certain frequency, or e) at only certain frequencies for a certain energy. QUICK QUIZ 40.2 ANSWER (d). The condition that the oscillators in a blackbody can have only discrete energy values according to Equation 40.4, En = nhf, does not imply that the oscillator may only oscillate at discrete frequencies. In fact, the blackbody spectrum is continuous precisely because all oscillation frequencies are allowed. What Equation 40.4 does imply is that if an oscillator is oscillating at a specific frequency, then it may only occupy certain discrete energy states, and that transitions are only allowed between these discrete energy states.