Surfaces * Gas adsorption at solid surfaces

Surfaces – Gas adsorption at solid surfaces
1/28/13
Why Surfaces?
• Heterogeneous catalysts are solids
– Solid surfaces can immobilize catalyst particles
• Reactions occur at interfaces
– Liquid/solid
– Gas/solid
• Atoms in the surface are under-coordinated compared to bulk atoms
– Fcc bulk = 12 bonds
• (111) = 9
• (100) = 8
• (110) = 6
– More reactive compared to the bulk
• On the nano-scale, surfaces become more important because higher surface : volume ratio
Definitions
• Adsorption: A molecule (adsorbate) that forms a bond to the surface (adsorbent).
– Associative Adsorption: A gas molecule adsorbs without fragmentation.
– Dissociative Adsorption: A gas molecule adsorbs and fragmentation occurs.
• Fractional coverage of adsorbate (θ)
N
S
N where N s
= number of surface sites occupied by adsorbate and N = total number of substrate adsorption sites
– When θ = 1, a monolayer exists
– θ = occupied sites, 1-θ = free sites
Heats of Adsorption
• Adsorption of gas on a solid is exothermic.
– Microcalorimetry a technique that measures heat given off due to molecular adsorption
Determining the thermal energy evolved when a known amount of gas is allowed to adsorb onto a clean surface (T rise of solid) can help to derive ΔH
AD
.
ΔG ° = -RTlnK ° = ΔH °
AD
– TΔS °
AD lnK ° = ΔH °
AD
/RT + ΔS °
AD
/R
A
( g )
S
K
A
S S
surface site
Differentiate with respect to T at constant θ
[
/
T(lnK ° )]
θ
= ΔH °
AD
/RT 2
P
2
T
2
P
1
T
1
Remember: KP = θ/(1-θ)
[
[
/
[
/
/
θ
θ
+ [
T(lnP)]
= [
/
/
T(lnP)]
T(lnK °
θ
= 0
)]
θ
θ
= ΔH
AD
/RT 2
[ln(P
1
/P
2
)]
θ
= (ΔH
AD
/R)(T
1
– T
2
)
• Isosteric (constant coverage) enthalpy of adsorption can be determined
Adsorbate Bonding
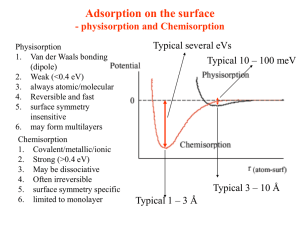
• Physisorption: Adsorbate/Adsorbent bonding interaction is long range, weak, and reversible, associated with van der
Waal interactions. There is a negligible exchange of electrons and a surface potential at the interface between two phases – gas and solid – where an
“overspill” of electron charge from the solid into the gas phase results in an imbalance of electron density. (ΔH ° <35 kJ/mole
• Chemisorption: An exchange of electrons between absorbate and adsorbent occurs, associated with covalent, ionic and metallic bonding. The ΔH
AD is larger than that of physisorption. As chemisorption proceeds, defects in ordered arrays and closer proximities of adsorbates destabilize the adsorbed layer causing disorder, and this is seen in ΔH
AD
.
Kinetics
• Hertz-Knudsen equation
– Flux of molecules
Z
W
p
2
mk
B
T
1/ 2
• Sticking probability (S) is the probability of a molecule being associatively adsorbed from the gas phase into a chemisorbed state assuming Langmuir behavior.
• S = S
0
(1-θ) where S is the rate of adsorption of adsorbates over the rate of collision of molecules with the surface (Z), and S
0 is the sticking probability at θ = 0.
• Sometimes S is larger than predicted using Langmuir isotherm because if a molecule hits a filled site, it could form weak Van der
Waal interactions with the surface, diffuse, and then move to a vacant spot where it will become chemisorbed. If adsorbate is initially physisorbed to a vacant site, it is referred to as an intrinsic precursor state, while if it is physisorbed to a filled site, it is an extrinsic precursor state.
Well-defined Surfaces
In order to make surfaces more comparable, controlled amounts of defects are added to surfaces.
Typically, single crystal surfaces are used – aligned using X-ray back scattering then polished so that a particular crystal plane is exposed. The surfaces are then
Flat = large terraces
Vicinal = small terraces with many steps flat, with mostly large terraces, or vicinal, with short, flat terraces that are separated by atomic steps.
Miller Indices
• The Miller index is used for notation of the crystal planes
– (x,y,z) for simple cubic, face centered cubic (fcc), or body centered cubic (bcc)
– (w,x,y,z) for hexagonal close packed surfaces (hcp)
• Rules
– Find where the plane intersects the x, y, and z axes in multiples of unit cell dimensions (a)
• is used for a plane parallel to an axis, and –1 is written as Ī
– Take the reciprocal of the numbers
– If fractions result, make the ratio into whole numbers.
– Planes with a high Miller index are not flat, but have narrow planes separated by steps and are described with the following
• n(x,y,z) x (u,v,w), where n = the average number of atoms on a terrace,
(x,y,z) is the Miller index of the plane and (u,v,w) is the Miller index of the step.
– fcc: (331) : 3(111) x (111), which is 3-atom wide (111) terraces separated by
(111) steps.
Examples
Z
Axes in unit cell dimensions
X
Y
Example: A plane that runs parallel to the x-axis, but intersects the y- and zaxes at 1:
, 1, 1 becomes (011).
Intercepts = 2, 3, 4
Reciprocal = 1/2, 1/3, 1/4
To get Miller index multiple by least common denominator (12)
Miller Index = (6,4,3)
Stereographic Triangle
• Every Miller Index is combination of (111), (100) and(110)
– Width of terrace; facets of terrace, step edge, and kink sites
• Stereographic Triangle is a 2D representation of 3D space – Highlights all surfaces of 1 handedness
• If Miller index “looks” like a high symmetry surface, the terraces are of that facet i.e – (10,10,9) has (111) terraces.
• Chiral Miller index (hkl) defined as h ≠ k ≠ l ≠ 0
• Chirality of surface denoted by direction of decreasing density of microfacets
• (111) > (100) > (110)
• CW = R CCW = S
• (111) = 1 specific face
• {111} = set of equivalent faces
• [111] = 1 specific direction
• <111> = set of equivalent directions
Surface Explorer
• http://surfexp.fhi-berlin.mpg.de/
• Allows input of any Miller Index to visualize the surface
Surface Relaxation
• Atoms in (111), (100), and (110) fcc have lost 3, 4 or 5 nearest neighbors of the original 12 and to compensate for the loss of bonding, the surface will relax, which is an oscillatory change in interplanar spacing (Δd).
• During surface relaxation, the 1 st layer of atoms contracts towards the 2 nd layer to increase coordination, and the 3 rd nd 2 oscillations are damped.
layer reacts by expanding away from the layer. This continues until 5 or 6 layers deep (the selvedge) until the
• The largest relaxation occurs in high energy surfaces with low atomic density.
– In fcc, (110) > (100) > (111)
– In bcc (111) > (100) > (110)
• If the energy is large enough, the plane could reconstruct to enhance coordination of surface atoms and to lower the energy of the surface.
Au 22x√3 (110) Si(111) 7x7
Preparation of a Clean Surface
Cleaning the surface
– Layered compounds (graphite, mica and some semiconductors) can be cleaved
– Metal catalysts can be rid of excess oxygen by heating the sample in the presence of hydrogen.
– Sputtering cleans the sample using high energy argon ions to bombard the surface. The energy transfer from the ions breaks bonds of the surface and adsorbates, but leaves the surface rough, so it is best to anneal the surface to flatten it after sputtering.
Sputtering and annealing should be repeated several times because annealing the sample can bring impurities from the bulk to the surface.
Maintenance of a Clean Surface
Surface bombardment (Z) by molecules
Z = P/(2πMkT) where P is P ambient
(Ncm -2 ), M is in units of (kg/molecule), T is in Kelvin, and k is Boltzmann’s constant (J/K). The rate of contamination also depends on a molecule’s sticking ability.
Example: CO – 300K, P = 10 -6 torr, and assume worst,
S = 1. Z = 3.82 x 10 14 cm -2 s -1 . If atomic density is typically 10 15 cm 2 ,
Z/(cm/ML) = 0.382 ML/s
And for 1 ML = 2.6 s to adsorb at an ambient P
However, at 10 -10 torr, it takes 7.3 hours for 1 ML to adsorb, so an ultra high vacuum is necessary to keep surfaces clean.
Langmuir units (L) are used to describe gas exposures, and the definition is an exposure for 1 second at 10 -6 torr.
Dosing Molecules
• Unit of exposure = Langmuir (L)
– 1 L = 1 x 10 -6 torr*sec (1.3 x 10 -6 mbar*sec)
– 0.01 L = 1 x 10 -8 mbar * 1 sec
– 0.05 L = 1 x 10 -8 mbar * 5 sec
– 0.05 L = 5 x 10 -8 mbar * 1 sec
– 0.12 L of MeOH at 30 K, 100 L of O
2 at RT, 10 3 L of H
2 onto Pd/Cu(111) at RT
• To compare Langmuirs to coverage (θ ML), need to do STM or spectroscopy
• When comparing Langmuir doses of different molecules need to account for ionization cross-section
– NIST website ( http://physics.nist.gov/PhysRefData/Ionization/Xsection.html
)
– Assume 100 eV to compare
Mobility in 2 Directions
• Surface Diffusion: atoms and molecules are mobile to equilibrate and minimize free energy states.
• The rate of diffusion is dependent on the direction in which the diffusion occurs, T and θ.
• Physisorbed molecules have low ΔH °
AD and low diffusion activation energies and therefore are very mobile in ambient T. (1/3 of Desorption Energy)
• Chemisorbed molecules, on the other hand, are immobile in ambient T, because of higher activation energies.
• The activation energy is larger for rough surfaces than for smooth surfaces. For the rate of diffusion: Stepped
< (110) < (100) < (111).
Lateral Interactions
• Direct Coulombic: (alkali metals) adsorbates and either adsorbent or oppositely charged adsorbates transfer charge and become coadsorbed.
• Direct Covalent/Metallic: (transition metals) two adsorbates with partially filled valence orbits bond together
• Van der Waal: (self-assembling ML (SAM)) distortions of adsorbate’s electron density induces a temporary dipole moment in a neighboring adsorbate. These interactions are dominant in nonpolar, large molecules.
• If substrate/adsorbate interactions are greater than adsorbate/adsorbate interactions, the overlayer is referred to as
“commensurate” and the interadsorbate spacing is equal to the substrate/adsorbate spacing or a multiple.
• If substrate/adsorbate interactions are similar to adsorbate/adsorbate interactions, the overlayer is referred to as
“incommensurate”, and the spacing may not be a multiple, and a wide range of sites will be occupied.
Langmuir Adsorption Isotherms
Adsorption Isotherm: θ depends on, and is linearly related to, pressure (P) at a constant temperature (T). This relationship is used to look at equilibrium adsorption behavior, and to find the total surface area of a substrate
(S
A
), but the following assumptions must be made:
θ
Low P
– 1: The solid surface is uniform and each site (all are equivalent) can be occupied by 1 molecule.
– 2: A dynamic equilibrium exists between the gas (at P) and the adsorbed layer at constant T. For associative adsorption: M(g) + S(surface site) k a
M-S (k a and k d are the rate constants of adsorption and desorption, k d respectively)
– 3: Adsorbates (g) continually collide with the surface. Upon impact of a vacant site, the adsorbate sticks. Upon impact of a filled site, they are reflected back into the gas phase.
– 4: Once adsorbed, molecules are localized and the enthalpy of adsorption (ΔH
AD
) per site is constant, independent of θ.
Langmuir Adsorption Isotherms continued
N
S
N
KP
1
KP
K
k k d a
Langmuir Adsorption Isotherms continued
Surface area, S
A
= N*A m
, where A m
= area molecule and
N can be calculated using m
: m
/M = n
M
= total # of moles in 1 monolayer (ML) n
M
= N/L, where L = Avogadro’s number so N = (m
L)/M, where M = molar mass or N can be calculated using V
:
PV
= n
M
RT (at constant P & T), so
N = (PV
L)/RT
And S
A as specific surface area = S
A
/mass of substrate
Kinetics
The rate at which molecule collide with the surface and lose energy to become adsorbed is measured by a thermal accommodation coefficient (α)
α = (T f
– T i
)/(T s
– T i
) where T i is the T initial of the molecule in the gas phase, T f is the T final of the molecule after collision with the surface, and T s is the T surface
.
When T i
= T f
, α = 0 and the molecules are elastically scattered.
When T s
= T f
, α = 1 and the adsorbates are all
“accommodated”.
King and Wells to Determine Sticking
Coefficient
pressure time s ( t )
p o p o
p ( t )
p b