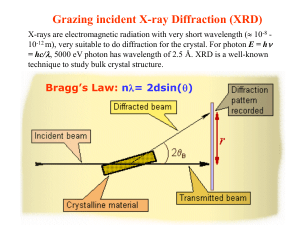
Mark Rivers: Introduction
advertisement

Advanced Photon Source GeoSoilEnviroCARS Operate a national user facility at the APS for the conduct of frontier experiments in earth, planetary, soil, and environmental sciences. Supported by DOE-Geosciences and NSF-Earth Sciences Techniques available to the scientific community • Microprobe and microspectroscopy • Microtomography • Surface scattering and spectroscopy • Microcrystal and powder diffraction • Energy-dispersive and monochromatic diffraction and spectroscopy in the diamond-cell • Energy-dispersive diffraction and imaging in a 250 ton multi-anvil press • Energy-dispersive and monochromatic diffraction in a 1000 ton press • Inelastic scattering in the diamond-cell Large Volume Press (LVP) High Pressure Research Beamline Scientist: Yanbin Wang Instruments: • 250 T LVP on bending magnet source • 1000 T LVP on undulator source Applications: • High resolution crystallography • Structures of glasses and melts • Phase equilibrium studies with in-situ P/T determination • Time resolved experiments on kinetics of reactions (sub-second) • Viscosity measurements by falling sphere technique 1000 ton press in Station ID-D Diamond Anvil Cell High Pressure Research Beamline Scientist: Guoyin Shen, Vitali Prakapenka Instruments: • Diamond Anvil Cell Diffractometer • X-ray microfocusing with KB mirrors • Double-sided heating with two YLF lasers • Optical spectrograph for temperature measurement • Brillouin spectrometer in 13-BM-D (new) • Raman spectrometer for pressure measurement Applications: • Very high pressure (to 360 GPa) • Temperature to 7000 K • Small sample, volume at high P-T • Iron at Earth’s core P-T conditions • Melting curves at high pressure • High P-T phase diagrams • Thermal EOS at high P Laser heated Diamond Anvil Cell Apparatus in Station ID-D Surface Scattering and Spectroscopy and Microcrystallography Beamline Scientists: Peter Eng, Matt Newville Instruments: • General purpose diffractometers for surface and microcrystal diffraction (2 instruments, 13-ID-C, 13-BM-C) • X-ray focusing with large KB mirrors • CCD and multi-element Ge detectors Applications: • Diffraction from water/mineral interfaces • Metal sorption to hydrated mineral surfaces • Identification of minerals in complex earth materials • Structural determination on microcrystals • Microcrystal structures under extreme conditions (pressure, temperature) • Structures of melts and glasses • Chemical speciation of atoms in specific lattice sites Surface Spectroscopy Apparatus in Station ID-C X-ray Fluorescence Microprobe: MicroXRF and MicroXAFS Beamline Scientists: Steve Sutton, Matt Newville Instruments: • X-ray microfocusing to 1 micrometer with KB mirrors • Multi-element solid state x-ray detector for high count rates • Wavelength dispersive spectrometer for high energy resolution applications • Fluorescence microtomography Applications: • Chemical speciation in heterogeneous materials • Compositions of buried components (fluid inclusions ) • Trace element partitioning studies • Compositional mapping (diffusion, sorption, zonation) • Compositions of microparticles (oceanic particulates, micrometeorites) X-ray No Microprobe ID-C style in Figure Error! text ofat Station specified document.-1: Downstream view of the X-ray Microprobe apparatus installed in station 13-IDC showing KB microfocusing system, optical Copper Speciation in Hydrothermal Fluid Inclusions J. Mavrogenes and A. Berry (Australian National University) • XAFS spectra identify the stable complexes as [Cu(OH2)6]2+ at 25˚C, [CuCl2]at 200˚C, and [CuCl(OH2)] at the homogenization temperature of around 400˚C. • Change in copper coordination and oxidation state is fully reversible. • First direct spectroscopic evidence for vapor-phase Cu speciation - suggest copper is transported in the vapor phase as a neutral chloride complex. Cu 25oC O Low Temperature 2.35Å Cu2+ O 1.96Å Cu 495oC Cl 2.09Å Cu1+ High Temperature (Mavrogenes, J.A., A.J. Berry, M. Newville, and S.R. Sutton (2001) Copper speciation in vapor phase fluid inclusions from the Mole Granite, Australia. Am. Mineral., submitted) Microtomography Beamline Scientists: Mark Rivers, Peter Eng Instruments: • Flood-field tomography (conventional CAT scan approach) • Fluorescence tomography (pencil beam; element specific) Applications: • CAT scans with micrometer resolution • Elemental specificity using edge tomography and fluorescence tomography • Dynamic studies of fluids in rocks and soils • Root-soil-micro-organism interactions • Micro-structure visualization of rare, precious and fragile objects (soil aggregates, plant tissue, meteorites, fossils) X-ray Tomograpy at Station BM-D Microtomography (CAT scan) • Same as a medical CAT scan, but with more than 100 times better spatial resolution • Allows one to see “inside” an object in 3-D without having to cut it • Works by reconstruction of cross sections from a set of “projections” or radiographs, just like normal medical x-ray images • Allows study of internal structure of objects which cannot be sectioned because they are: – Too valuable – Too fragile – Too time-consuming Absorption Tomography • Fast – Typically 720 projections to create a 650x650x520 voxel image – 10-30 minutes • Examples: – Eocene snail fossil, 20 mm tall movie – Pumice sample Movie • Identification of glass, quartz, feldspar and oxides possible from known compositions and measured attenuation coefficients – Hydrous glass vesiculation (Don Baker, McGill) • Radiography with furnace (movie) • Tomography after quench (movie) Differential Absorption Tomography • Collect 2 absorption data sets, above and below the absorption edge of the element of interest – also fast • Requires a substantial change in linear attenuation coefficient due to element of interest – Major elements, not trace elements • Example: 2mm capillaries with KI solutions, varying concentration 33.1 keV, below I edge 33.2 keV, above I edge Difference Differential Absorption Tomography 8mm diameter sand column with aqueous phase containing Cs and organic phase containing I. (Clint Willson, LSU) 32.5 keV, below I and Cs K absorption edges 33.2 keV, above I and below Cs K absorption edges 33.2 - 32.5 keV, showing distribution of I in the organic phase 36.0 keV, above I and Cs K absorption edges 36.0 - 33.2, showing distribution of Cs in the aqueous phase