Complex Reactions
advertisement

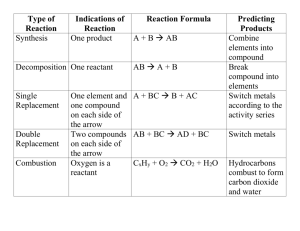
Sequential Reactions and Intermediates (25.7) • Sequential reactions (elementary) involve multiple reactions in which one or more intermediates are formed – The differential form of the rate is written with respect to product formation – Rate law only involves intermediate since it is the only species that generates products kA kI A I P Rate d P k I I dt • Intermediate concentration is extremely hard to measure, so we need to we can measure relate it to quantities – We should be able to measure reactant concentration, so we rely on the fact that A only decays in one way dA k A A dt A A0 ek t A • Since the intermediate only formsfrom the reactant, we can express its concentration in terms of A – The first reaction creates I, the second reaction depletes it (hence the negative sign) dI k A A k I I dt I kA ekA t ek I t A0 kI k A Sequential Reactions and Rate Determining Steps (25.7) • Concentration of product can be determined using a simple mass balance principle – The sum of concentrations of all substances at any time must be equal to the initial concentration of reactant A0 A I P kA ek I t kI ekA t 1A0 P kI kA • The size of the rate constants is an indication of how quickly each part of the reaction proceeds – If kI is much larger than kA, the intermediate does not last very long and thus does not build up in the reaction – If kA is much larger than kI, then the reactant decomposes quickly and a significant amount of intermediate will form • The rate-determining (or rate-limiting) step is the slowest step in the mechanism and it dictates how quickly products will form – If first step is rate-limiting, the reaction looks like it is one step (i.e., decay of reactant and formation of product follow first order kinetics) – If second step is rate-limiting, reaction follows first-order for intermediate Parallel Reactions (25.8) • Parallel reactions involve the reactant decaying into more than one product – The rate of decay of the reactant is related to the rate constants of both processes kB kC B A C d A k B B kC C dt A A0 ek B kC t • The rates of formation of each product has a simple form due to the simplicity of the differential rate equation – The product concentration (P = B or C) differs based on the rate constant dP k P A k P A0 ekB kC t dt P kP A0 1 ekB kC t kB kC • The to the ratio of the rate constants for ratio of concentrations is related a parallel set of reactions – The overall yield (ϕ) of a product in the reaction is the ratio of the concentration of the product of interest over the sum of all product concentrations B kB C kC k1 k1 k 2 k 3 ... Reversible Reactions and Equilibrium (25.10) • Reversible reactions are ones in which the reactants can be generated from products – Each direction of the reaction has a rate constant associated with it kA A B kB Rate dA d B kA A k B B dt dt • If the reaction starts with only A, after a certain length of time the concentrations of A and B stabilize (i.e., equilibrium is obtained) Aeq A0 kB B A 1 eq 0 kA kB kB kA kB • At equilibrium, rate of change of A and B are zero – Equilibrium constant (K) can be expressed as a ratio of rate constants dAeq dt dBeq dt kA Aeq kB Beq 0 kA Beq K kB Aeq Concentration Profile for Sequential Reactions Rate-Limiting Behavior in Sequential Reactions Concentration Profile for Parallel Reactions Concentration Profile for Reversible Reaction