Lecture_6_The thermo..
advertisement
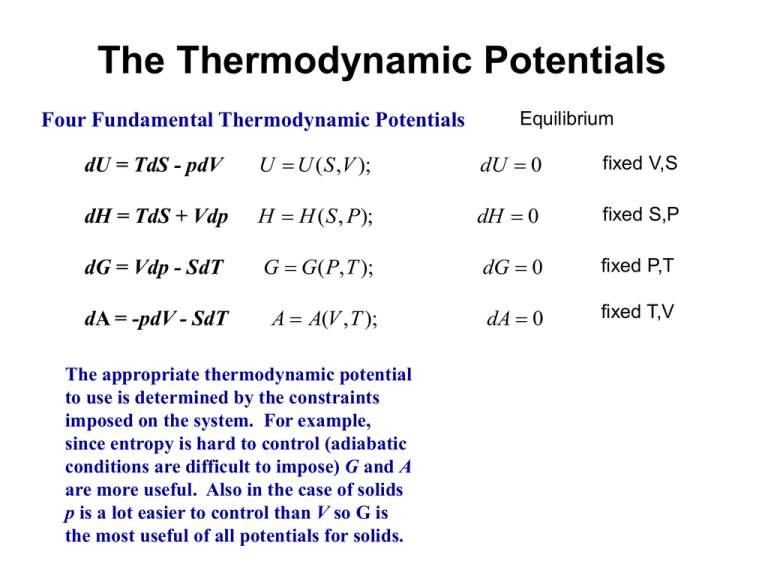
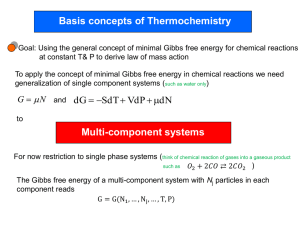
The Thermodynamic Potentials Four Fundamental Thermodynamic Potentials Equilibrium dU = TdS - pdV U U ( S ,V ); dU 0 fixed V,S dH = TdS + Vdp H H ( S , P); dH 0 fixed S,P dG = Vdp - SdT G G( P, T ); dG 0 fixed P,T dA = -pdV - SdT A A(V , T ); dA 0 fixed T,V The appropriate thermodynamic potential to use is determined by the constraints imposed on the system. For example, since entropy is hard to control (adiabatic conditions are difficult to impose) G and A are more useful. Also in the case of solids p is a lot easier to control than V so G is the most useful of all potentials for solids. Our discussion of these thermodynamic potentials has considered only “closed” (fixed size and composition) systems to this point. In this case two independent variables uniquely defines the state of the system. For example for a system at constant P and T the condition, dG = 0 defines equilibrium, i.e., equilibrium is attained when the Gibbs potential or Gibbs Free Energy reaches a minimum value. If the composition of the system is variable in that the number of moles of the various species present changes (e.g., as a consequence of a chemical reaction) then minimization of G at fixed P and T occurs when the system has a unique composition. For example, for a system containing CO, CO2, H2 and H2O at fixed P and T, minimization of G occurs when the following reaction reaches equilibrium. CO H 2O CO2 H 2 Since G is an EXTENSIVE property, for multi-component or open system it is necessary that the number of moles of each component be specified. i.e., G G T , P, n1 , n2 , n3 ,...ni G dni i 1 ni T , P , n j ... k Then G G G G dG dT dP dn1 dn2 ... T P , n1 , n2 ... P T , n1 , n2 ... n1 P ,T , n2 , n3 ... n2 P ,T , n1 , n3 ... If the number of moles of each of the individual species remain fixed we know that dG SdT VdP G S ; T P , n1 , n2 ... G V P T , n1 , n2 ... G dG SdT VdP dni i 1 ni T , P , n j ... k G i Gi Chemical potential: The quantity ni T ,P ,n j ... is called the chemical potential of component i. It correspond to the rate of change of G with ni when the component i is added to the system at fixed P,T and number of moles of all other species. k dG SdT VdP i dni i 1 One can add the same open system term, i dni for the other thermodynamic potentials, i.e., U, H and A. The chemical potential is the partial molal Gibbs Free Energy (or U,H, A) of component i. Similar equations can be written for other extensive variables, e.g., V V dV dn dn2 ... 1 n1 P ,T , n2 , n3 ... n2 P ,T , n1 , n3 ... dV V1dn1 V2 dn2 ... Physically this corresponds to how the volume in the system changes upon addition of 1 mole of component ni at fixed P,T and mole numbers of other components. Maxwell relations: These mathematical relations are used to connect experimentally measurable quantities to those that are not easily accessible Consider the relation for the Gibbs Free Energy: dG SdT VdP at fixed T G V P T at fixed P G S T P Now take the derivative of these quantities at fixed P and T respectively, G V T P T P T P G S P T P T P T G V T P T P T P G S P T P T P T If we compare the LHS of these equations, they must be equal since G is a state function and an exact differential and the order of differentiation is inconsequential, G G T P P T T P P T So, the RHS of each of these equations must be equal, S V P T T P Similarly we can develop a Maxwell relation from each of the other three potentials: A S V P T T P B S P V T T V C T V P S S P D T P V S S V Let’s see how these Maxwell relations ca be useful. Consider the following for the entropy. S S T , V S S dS dT dV T V V T Using the definition of the constant volume heat capacity and the definition of entropy for a reversible process q dU CV dT dT V V TdS qrev dU ncV dT dS qrev T Dividing by dT, the entropy change with temperature at fixed P is ncV S T T V Then, S S dS dT dV T V V T dS ncV S dT dV T V T For the entropy change with volume at fixed T we can use the Maxwell relation B S P V T T V dS ncV P dT dV T T V Now from the ideal gas law, PV = nRT nR P T V V dS ncV nR dT dV T V Integrating between states 1 and 2, T2 S2 S1 ncV ln T1 V2 nR ln V1 This equation can be used to evaluate the entropy change at fixed T, problem 4.1. Some important bits of information For a mechanically isolated system kept at constant temperature and volume the A = A(V, T) never increases. Equilibrium is determined by the state of minimum A and defined by the condition, dA = 0. For a mechanically isolated system kept at constant temperature and pressure the G = G(p, T) never increases. Equilibrium is determined by the state of minimum G and defined by the condition, dG = 0. Consider a system maintained at constant p. Then G SdT T2 T2 T1 T1 S S T dT C p T d ln T T2 S T2 S T1 C p T d ln T T1 T2 T2 G G T2 G T1 T2 T1 S T1 dT C p T d ln T T T1 1 Temperature dependence of H, S, and G Consider a phase undergoing a change in temp @ const P q dH Cp dT p dT p dS q T and C p dT T C p d ln T dG Vdp SdT @ Const. P dG SdT H C p ( t )dT S C p (T )d lnT Temperature dependence of H, S, and G T2 G S(T )dT T1 S C p (T ) d ln T T2 S (T2 ) S (T1 ) C p (T )d ln T T1 T2 T2 G G(T2 ) G(T1 ) T2 T1 S (T1 ) dT C p (T )d ln T T T1 1 T2 G T S( T1 ) SdT T1 Temperature Dependence of the Heat Capacity Cp Dulong and Petit value 1 CV 3R T (K) Contributions to Specific Heat 1. 2. 3. 4. 5. 6. Translational motion of free electrons ~ T1 Lattice vibrations ~ T3 Internal vib. within a molecule Rotation of molecules Excitation of upper energy levels Anomalous effects Temp. dep. of H, S, and G H S S0 ≡ 0 pure elemental solids, Third Law T H0 298K T ref. state for H is arbitrarily set@ H(298) = 0 and P = 1 atm for elemental substances H G H TS H G G0 slope = Cp T T TS G G slope = -S Thermodynamic Description of Phase Transitions Gs H s TSs 1. Component Solidification G Gl Hl TSl LT Tm G G(T ) Gs (T ) Gl (T ) H TS @ T= Tm Gsolid Gl =Gs dGl = dGs ; G = 0 Gliquid T* Tm T ΔS = H Tm L Tm Where L is the enthalpy change of the transition or the heat of fusion (latent heat). For a small undercooling to say T* ΔH and ΔS are constant (zeroth order approx.) ΔG H TS H T ( H ) Tm T* L ΔG H(1 ) T Tm Tm Where ΔT = T m – T* * Note that L will be negative The location of the transition temp Tm will change with pressure dGl Vl dp Sl dT dGs Vs dp Ss dT dGl dGs dp Sl S s S dT Vl Vs V dp L dT Tm V L S Tm Clapeyron equation For a small change in melting point ΔT, we can assume that ΔS & ΔH are constant so TV T P H The Clapeyron eq. governs the vapor pressure in any first order transition. Melting or vaporization transitions are called first-order transitions (Ehrenfest scheme) because there is a discontinuity in entropy, volume etc which are the 1st partial derivatives of G with respect to Xi i.e., G G S ; V T p p T S 0 V 0 There are phase transition for which ΔS = 0 and ΔV = 0 i.e., the first derivatives of G are continuous. Such a transition is not of first-order. According to Ehrenfest an nth order transition if at the transition point. nG1 nG2 n T T n Whereas all lower derivatives are equal. There are only two transitions known to fit this scheme gas – liquid 2nd order trans. in superconductivity Notable exceptions are; Curie pt. trans in ferromag. Order-disorder trans. in binary alloys λ – transition in liquid helium