Lecture 5
advertisement
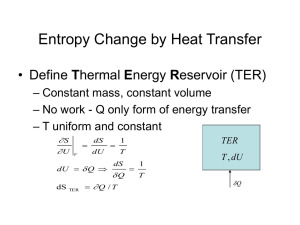
7.6 Entropy Change in Irreversible Processes • It is not possible to calculate the entropy change ΔS = SB - SA for an irreversible process between A and B, by integrating dq / T, the ratio of the heat increment over the temperature, along the actual irreversible path A-B characterizing the process. • However, since the entropy is a state function, the entropy change ΔS does not depend on the path chosen.The calculation of an irreversible process can be carried out via transferring the process into many reversible ones: • Three examples will be discussed here: (1) heat exchange between two metal blocks with different temperatures; (2) Water cooling from 90 to a room temperature; (2) A falling object. 7.7 Free Expansion of an Ideal Gas 7.8 Entropy Change for a Liquid or Solid Chapter 8 Thermodynamics Potential 8.1 Introduction • Thermodynamic potentials: Helmholtz function F and the Gibbs function G. • The enthalpy, Helmholtz function and Gibbs functions are all related to the internal energy and can be derived with a procedure known as Legendre differential transformation. • The combined first and second laws read dU = Tds – PdV where T and S, and -P and V are said to be canonically conjugate pairs. • By assuming U = U(S,V), one has 8.2 The Legendre Transformation • Consider a function Z = Z(x, y), the differential equation is dZ = Xdx + Ydy where X and x, Y and y are by definition canonically conjugate pairs. • We wish to replace (x, y) by (X, Y) as independent variables. This can be achieved via transforming the function Z(x,y) into a function M(X,Y). • Assume M(X,Y) = Z(x,y) – xX – yY Then dM = dZ – Xdx – xdX –Ydy – ydY dM = -xdX - ydY 8.3 Definition of the Thermodynamic Potentials 8.4 The Maxwell Relations 8.5 The Helmholtz Function • The change in internal energy is the heat flow in an isochoric reversible process. • The change in enthalpy H is the heat flow in an isobaric reversible process. • The change in the Helmholtz function in an isothermal reversible process is the work done on or by the system. • The decrease in F equals the maximum energy that can be made available for work. 8.6 The Gibbs Function • Based on the second law of thermodynamics dQ ≤ T∆S with dQ = ∆U + P ∆V • Combine the above expressions ∆U + P ∆V ≤ T∆S ∆U + P ∆V - T∆S ≤ 0 • Since G = U + PV –TS (∆G)T,P≤ 0 at constant T and P or G f ≤ Gi • Gibbs function decreases in a process until a minimum is reach, i.e. equilibrium point. • Note that T and P need not to be constant throughout the process, they only need to have the same initial and final values. 8.7 Application of the Gibbs Function to Phase Transitions 8.8 An application of the Maxwell Relations