Radium 223 - The Prostate Net
advertisement

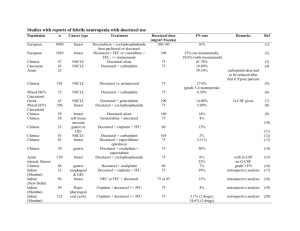
Tratamiento en Cáncer de Próstata en progresión con niveles de castración de testosterona (CPRC) Dr Pablo Maroto Hospital de Sant Pau Natural History of Prostate Cancer Androgen Deprivation Chemotherapy Death Local Therapy Therapies After LHRH Agonists and Antiandrogen Under the care of Symptomatic ONCOLOGIST Asymptomatic Non Metastatic Castration Sensitive Post Chemo Metastatic Castration Resistant • Typical presentation of patients as they move through the different stages. The line represents level burden of disease. Time is not proportional Abbreviation: LHRH=luteinizing hormone-releasing hormone. Phase III Docetaxel Studies in CRPC Demonstrating Survival Benefit TAX 327 N = 1,006 RA ND O MI S E Mitoxantrone 12 mg/m2 Prednisone 10 mg q day Q 21 days up to 10 cycles Docetaxel 30 mg/m2/wk Prednisone 10 mg q day 5 on; 1 off x 6 cycles Docetaxel 75 mg/m2 Prednisone 10 mg q day Q 21 days up to 10 cycles SWOG 9916 N = 770 aWarfarin and aspirin. SWOG = Southwest Oncology Group. Tannock et al, 2004; Petrylak et al, 2004. RA ND O MI S E Mitoxantrone 12 mg/m2 Prednisone 5 mg bid Q 21 days Docetaxel 60 mg/m2 D2 Estramustine 280 mg D1–5a Dexamethasone 20 mg, tid D1–2 Opciones de tratamiento en CPRC en segunda línea • Prevención de EREs • Segundas líneas hormonales – – Abiraterona Enzalutamida • Quimioterapia: Cabazitaxel • Con enfermedad predominantemente ósea – Radioisótopos: Radium 223 RANKL: Mediador en el “Círculo Vicioso” de destrucción ósea de la metástasis RANKL RANK Tumor Cell PTHrP, BMP, TGF-β, IGF, FGF, VEGF, ET1, WNT PDGF, BMPs TGF-β, IGFs FGFs Activated Osteoclast Osteoblasts Adapted from Roodman D. N Engl J Med. 2004;350:1655. Denosumab interrumpiría el “Círculo Vicioso” RANKL RANK Tumor Cell PTHrP, BMP, TGF-β, IGF, FGF, VEGF, ET1, WNT Formation Inhibited Denosumab PDGF, BMPs TGF-β, IGFs FGFs Osteoblasts Adapted from Roodman D. N Engl J Med. 2004;350:1655. Apoptotic Osteoclast Study Design: International, Randomized, Double-Blind, Active-Controlled Study Key Inclusion • Hormone-refractory (castration resistant) prostate cancer and bone metastases Key Exclusion • Current or prior IV bisphosphonate treatment Denosumab 120 mg SC and Placebo IV* every 4 weeks (N = 950) Zoledronic acid 4 mg IV* and Placebo SC every 4 weeks (N = 951) • Calcium and Vitamin D supplemented in both treatment groups • Accrual period from May 2006 to December 2008 • Analysis cut-off date October 2009 *Per protocol and Zometa® label, IV product dose adjusted for baseline creatinine clearance and subsequent dose intervals determined by serum creatinine. No SC dose adjustments made due to increased serum creatinine. Time to First and Subsequent On-Study SRE* (Multiple Event Analysis) Cumulative Mean Number of SREs per Patient 2.0 Rate Ratio = 0.82 (95% CI: 0.71, 0.94) P = 0.008 1.8 18% Risk Reduction 1.6 1.4 1.2 1.0 0.8 0.6 Events 0.4 Denosumab Zoledronic acid 0.2 0.0 0 3 6 9 12 15 18 21 24 Month *Events occurring at least 21 days apart 27 494 584 30 33 36 TROPIC: Cabazitaxel vs Mitoxantrone ¿Es importante una mCRPC patients who progressed during and rama control after treatment with a docetaxel-based regimen (N=755) con mitoxantrone? Stratification factors ECOG PS (0, 1 vs. 2) • Measurable vs. non-measurable disease cabazitaxel 25 mg/m² q 3 wk + prednisone* for 10 cycles (n=378) mitoxantrone 12 mg/m² q 3 wk + prednisone* for 10 cycles (n=377) *Oral prednisone/prednisolone: 10 mg daily. Primary endpoint: OS Secondary endpoints: Progression-free survival (PFS), response rate, and safety Inclusion: Patients with measurable disease must have progressed by RECIST; otherwise must have had new lesions or PSA progression Primary Endpoint: Overall Survival (ITT Analysis) Proportion of OS (%) MP 100 CBZP Median OS (months) 15.1 12.7 Hazard Ratio 80 0.70 0.59–0.83 95% CI P-value <.0001 60 40 20 0 Number at risk 0 months 6 months 12 months 18 months 24 months 30 months MP 377 300 188 67 11 1 CBZP 378 321 231 90 28 4 Factor Hazard ratio (95% CI) All patients 0.70 (0.59–0.83) ECOG status: 0,1 0.68 (0.57–0.82) ECOG status: 2 0.81 (0.48–1.38) Measurable disease: No 0.72 (0.55–0.93) Measurable disease: Yes 0.68 (0.54–0.85) No. of prior chemo: 1 0.67 (0.55–0.83) No. of prior chemo: ≥2 0.75 (0.55–1.02) Age: <65 0.81 (0.61–1.08) Age: ≥65 0.62 (0.50–0.78) Rising PSA: No 0.88 (0.61–1.26) Rising PSA: Yes 0.65 (0.53–0.80) Total docetaxel dose: <225 mg/m² 0.96 (0.49–1.86) Total docetaxel dose: ≥225 to 450 mg/m² 0.60 (0.43–0.84) Total docetaxel dose: ≥450 to 675 mg/m² 0.83 (0.60–1.16) Total docetaxel dose: ≥675 to 900 mg/m² 0.73 (0.48–1.10) Total docetaxel dose: ≥900 mg/m² 0.51 (0.33–0.79) Progression: During last docetaxel treatment 0.65 (0.47–0.90) Progression: <3 months since last docetaxel dose 0.70 (0.55–0.91) Progression: ≥3 months since last docetaxel dose 0.75 (0.51–1.11) favors CBZP 0 0 0.5 1 0.5 1 | favors MP 1.5 10 1.5 2 2 Most Frequent Grade ≥3 Treatment-Emergent AEs* Safety Population CBZP (n=371) MP (n=371) All grades (%) Grade ≥3 (%) All grades (%) Grade ≥3 (%) Any adverse event Febrile neutropenia Diarrhea 88.4 39.4 95.7 57.4 1.3 1.3 7.5 7.5 10.5 0.3 46.6 6.2 3 36.7 4.9 2.4 20.5 4.6 3 16.2 3.8 ¿Cómo condiciona 27.5 el tratamiento Asthenia 12.4 la toxicidad? Back pain 12.1 Fatigue Nausea 22.9 0.3 34.2 1.9 Vomiting 10.2 0 22.6 1.9 Hematuria 3.8 0.5 16.7 1.9 Abdominal pain 3.5 0 11.6 1.9 VOLUMEN 26, Nº28 2008 Prostate Cancer: Moving Forward by Reinventing the Wheel ... But This Time It Is Round Resistance to castration: is there still a way to play with hormonal drugs 3 4 1 2 Scher H et al, J Clin Oncol 2005 LHRH analogues Antiandrogens LHRH LHRH Analogue LHRH Brain Brain Pituitary Pituitary LH LH ACTH Adrenal Gland Testis Adrenal Gland Testis Testosterone Testosterone ACTH Androgens Androgens Antiandrogen Prostate Cancer Prostate Cancer Abiraterone: Inhibición síntesis teste, adrenal, ¿intratumoral? LHRH MW = 391.55 Brain RO Pituitary LH 3β-Acetoxy-17-(3-pyridyl)androsta-5,16-diene Inhibitor ACTH Adrenal Gland Testis Testosterone N Androgens Inhibitor? Prostate Cancer ? De novo synthesis CYP17 blockade inhibits androgen synthesis The Effects of MDV3100 on the Androgen Receptor Are Distinct from Bicalutamide Ligand LBD HSP 90 1. AR Binding Affinity 1 HD DBD • • • DHT ~ 5nM Bicalutamide ~160 nM MDV3100 ~35 nM 2. Nuclear Import • • • NTD 2 DHT: Bicalutamide: MDV3100: ++++ ++++ ++ 3. DNA Binding • • • 4 DHT: Bicalutamide: MDV3100: ++++ ++ - POL II 4. Coactivator recruitment • • • 3 DNA DHT: Bicalutamide: MDV3100: ++++ ++ - COU-AA-301: Fase III postquimioterapia ¿Influye en la Fase III, multicéntrico, decisión de tratar aleatorizado, doble ciego, para estudiar los beneficios clínicos de abiraterona junto a prednisona, en pacientes la necesidad con de cáncer de próstata metastásico que han progresado tras uno o prednisona? Pacientes •N=1195 •1 o 2 regímenes de QT previa, uno de ellos docetaxel ALEATORIZACIÓN 2:1 dos regímenes de quimioterapia. Abiraterona 1000 mg/día Prednisona 10 mg/día N=797 Objetivo principal •OS Objetivos secundarios Placebo Prednisona 10 mg/día N=398 •TTPP •rPFS •Respuesta PSA Gráfico extraído de: de Bono, J.S. et al. Abiraterone and increased survival in metastasic prostate cancer. NEJM: 2011;364(21):1995-2005. ¿Metástasis viscerales también? ¿Tiene importancia la respuesta por PSA? Diseño del estudio ALSYMPCA TRATAMIENTO PACIENTES ESTRATIFICACIÓN • Confirmed symptomatic CRPC • ≥ 2 bone metastases • No known visceral metastases • Total ALP: < 220 U/L vs ≥ 220 U/L • Bisphosphonate use: Yes vs No • Prior docetaxel: Yes vs No • Post-docetaxel or unfit for docetaxel R A N D OM I S E D 2:1 N = 922 Planned follow-up is 3 years Clinicaltrials.gov identifier: NCT00699751. 6 injections at 4-week intervals Radium-223 (50 kBq/kg) + Best standard of care Placebo (saline) + Best standard of care ALSYMPCA: Supervivencia Global 100 HR 0.695; 95% CI, 0.552-0.875 P = 0.00185 90 80 70 60 % Radium-223, n = 541 Median OS: 14.0 months 50 40 30 Placebo, n = 268 Median OS: 11.2 months 20 10 0 Month 0 3 6 9 12 15 18 21 24 27 Radium- 541 223 450 330 213 120 72 30 15 3 0 Placebo 268 218 147 89 49 28 15 7 3 0 ALSYMPCA: Tiempo al primer SRE 100 HR 0.610; 95% CI, 0.461-0.807 P = 0.00046 90 80 % Without SRE 70 Radium-223, n = 541 Median: 13.6 months 60 50 40 Placebo, n = 268 Median: 8.4 months 30 20 10 0 Month 0 3 6 9 12 15 18 21 Radium223 541 379 214 111 51 22 6 0 Placebo 268 159 74 30 15 7 2 0 ALSYMPCA: Efectos adversos de interés All Grades Grades 3 or 4 Radium-223 n (%) Placebo n (%) Radium-223 n (%) Placebo n (%) 136 (27) 69 (27) 54 (11) 29 (12) Neutropenia 20 (4) 2 (1) 9 (2) 2 (1) Thrombocytopenia 42 (8) 14 (6) 22 (4) 4 (2) Bone pain 217 (43) 147 (58) 89 (18) 59 (23) Diarrhoea 112 (22) 34 (13) 6 (1) 3 (1) Nausea 174 (34) 80 (32) 8 (2) 4 (2) Vomiting 88 (17) 32 (13) 10 (2) 6 (2) Constipation 89 (18) 46 (18) 6 (1) 2 (1) Haematologic Anaemia Non-Haematologic Resumen: Resultados Ensayos fase III en CPRC Agent (trial, year) Disease State Comparator Hazard Ratio P value Radium-223 (ALSYMPCA 2011) Symptomatic Bone metastases Placebo 0.695 0.00185 Docetaxel1 (TAX327 2004) Chemo-naive Mitoxantrone Prednisone 0.76 0.009 Cabazitaxel2 (TROPIC 2010) Post-docetaxel Mitoxantrone Prednisone 0.70 <0.0001 Enzalutamide3 (AFFIRM 2012) PostDocetaxel Placebo 0.63 0.0001 Abiraterone4 Post-docetaxel Placebo Prednisone 0.65 <0.001 (COU-AA-301 2010) No hay criterios definitorios para recomendar un tratamiento para un paciente dado ¿Seguro? PMR: 60 a. ECOG 0. Gleason 9. Respuesta hormona previa <12 m. Metástasis hepáticas. PSA de 19. Respuesta docetaxel intervalo 2 meses sin toxicidades relevantes JMR: 70 a. ECOG 0. Gleason 7. Respuesta hormona previa 19 m. Metástasis Óseas. PSA de 198. Respuesta docetaxel intervalo 4 meses Definir un algoritmo similar para hormonoterapia de rescate GRACIAS