N Engl J Med
advertisement
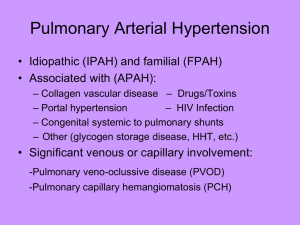
The Health Economics of PAH Moderator Harrison (Hap) Farber, MD Professor of Medicine Director Pulmonary Hypertension Center Boston University/Boston Medical Center Boston, Massachusetts Panelists R. James White, MD, PhD Associate Professor of Medicine Pharmacology and Physiology University of Rochester School of Medicine Rochester, New York PAH Diagnostic Workup General Considerationsa,b • Presentation/history suggestive of PH – Echocardiogram – ECG, chest radiograph, TTE, PFT, HRCT – V/Q scan – RHC • Presence of comorbidities (eg, diabetes, obesity, CAD); echocardiogram suggestive of PH diastolic dysfunction? – Initiate empiric loop diuretics – Sleep study undiagnosed sleep apnea? a. Galie N, et al. Eur Heart J. 2009;30:2493-2537[2]; b. McGoon et al. Chest. 2004;126(1 suppl):14S-34S.[3] When to Perform an RHC?a,b • Patient responding to empiric therapy: loop diuretics, apnea therapy? – Yes no further testing (ie, probable diastolic dysfunction) – No comprehensive guideline-recommended workup vs immediate RHC? • • • Prior history and diagnostic workup (ie, referral population) Rule out left heart disease, sleep apnea Immediate RHC is more cost-effective vs complete workupc – mPAP ≥ 25 mm Hg; PWP ≤ 15 mm Hg proceed with guideline-directed workup a. Galie N, et al. Eur Heart J. 2009;30:2493-2537[2]; b. McGoon et al. Chest. 2004;126(1 suppl):14S-34S[3]; c. Taylor B, et al. J Heart Lung Transplant. 2013;32:137-138.[1] Cost of Hospitalizations in PAH Registry and Trial Data • REVEAL Registrya – Mean total hospital days in the year after first admission for all pts with ≥ 1 hospitalization: 15.3 days (median: 7.0 days) – Estimated 2012 total costs (inpatient and outpatient) for PAH: $188 million • Estimated average cost/hospitalizationb – ~ $60,000-$100,000 (average 3-night stay on telemetry unit) • AMBITION datac – NNT to prevent 1 hospitalization: 9 – QALY: ~ $90,000-$100,000 or higher for longer hospital stays? – Combination therapy for shorter, less intense hospitalizations? a. Burger CD, et al. Chest. 2014;146:1263-1273[4]; b. Johnson S, et al. J Med Econ. 2013;16:14141422[5]; c. Galiè N. ERS 2014. Abstract 2916.[6] PAH Inpatient Management • Echocardiogram • RHC • CT scan • IV inotropes, prostacyclins • Anticoagulants • Supplemental oxygen, CPAP • Continuous monitoring McGoon M, et al. Mayo Clin Proc. 2009;84:191-207.[3] Reducing Hospitalizations Impact of Current and Emerging Therapies • Macitentan (ERA): SERAPHIN triala,b – – – Included pts on macitentan monoand combination therapy (PDE-5 inhibitors, oral or inhaled prostanoids, CCBs, l-arginine) Macitentan reduced primary end point (composite of death, atrial septostomy, lung transplantation, initiation of treatment with IV or SC prostanoids, worsening PAH) by 30%-45% (dose dependent; P = .01; P < .001) Reduced all-cause hospitalization by 32% (HR, 0.677; P = .0051) • Ambrisentan (ERA) ± tadalafil (PDE-5 inhibitor) vs monotherapy: AMBITION Trialc – Reduced clinical failure events by 50% (HR, 0.502; P = .0002); superior to each individual monotherapy (P < .01) main treatment effect driven by hospitalizations • Selexipag (selective IP receptor agonist): GRIPHON top-line datad – 80% of pts receiving oral PAH therapy at onset – Reduced morbidity/mortality event vs placebo by 39% (P < .0001) a. Pulido T, et al. N Engl J Med. 2013;369:809-818[8]; b. Mehta S, et al. ATS 2014. Abstract B17[9]; c. Galiè N, et al. ERS 2014. Abstract 2916[6]; d. Actelion press release.[10] Early, Aggressive PAH Therapy Effect on Outcomes • Hospitalization – Number – Duration – Utilization of resources • Patient satisfaction/QOL – Hospital-acquired infections/conditions • Morbidity and mortality events a. Pulido T, et al. N Engl J Med. 2013;369:809-818[8]; b. Mehta S, et al. ATS 2014. Abstract B17[9]; c. Galiè N, et al. ERS 2014. Abstract 2916[6]; d. Actelion press release [website].[10] The Real Cost of PAH Drugs Oral Treprostinila,b History of expensive PAH drugs • IV epoprostenol/SC treprostinil: ~ $90,000/year • Bosentan: ~ $80,000/year • Ambrisentan: ~ $80,000/year • Oral treprostinil – ~ $500,000/year [12 mg, three times daily patients transitioning from parenteral treprostinil (ongoing trialc)] – Compared with placebo: improved 6MWD, Borg dyspnea score (intent-to-treat population 26.0 m; P = .0001)d a. McLaughlin VV, et al. Circulation. 2009;119:2250-2294[11]; b. Frumkin LR, et al. Pharmacol Rev. 2012;64(3):583-620.[12]; c. White RJ, et al. ATS 2013. Abstract B64.[13]; d. Jing ZC, et al. Circulation. 2013;127:624-633[14] The Real Cost of PAH Drugs Riociguat Soluble guanylate cyclase stimulator • PATENT-1, randomized, phase 2 trial (PAH)a – Significant improvement • 6MWD • PVR (P < .001) • NT-proBNP levels (P < .001) • WHO FC (P = .003) • TTCW (P = .005) • Borg dyspnea score (P = .002) • CHEST-1, randomized, phase 3 trial (CTEPH)b – Significant improvement in • PVR (P < .001) • NT-proBNP level (P < .001) • WHO FC (P = .003) • Cost: $7500/monthc – Compared with tadalafil or generic sildenafil ($10,000-$12,000/year)d a. Ghofrani HA, et al. N Engl J Med. 2013;369:330-340[14]; b. Ghofrani HA, et al. N Engl J Med. 2013;369:319-329[16]; c. Walker T. Drug Topics. October 10, 2013[17]; d. McLaughlin VV, et al. Circulation. 2009;119:2250-2294.[11] Conclusions • Cost-effectiveness of PAH therapies will continue to be an issue, despite their improving efficacy • Efficacy, tolerability, and ease of use have to be balanced with costs to payers (ie, insurers, employers, government agencies) Abbreviations 6MWD = 6-minute walk distance CAD = coronary artery disease CCB = calcium channel blocker CPAP = continuous positive airway pressure CT = computed tomography CTEPH = chronic thromboembolic pulmonary hypertension ECG = echocardiogram ERA = endothelin receptor antagonist FDA = US Food and Drug Administration HR = hazard ratio HRCT = high-resolution computer tomography IP = intraperitoneal mPAP = mean pulmonary artery pressure NNT = number needed to treat NT-proBNP = N-terminal pro-brain natriuretic peptide PAH = pulmonary arterial hypertension PFT = pulmonary function test PH = pulmonary hypertension Abbreviations (cont) PVR = pulse volume recording QALY = quality-adjusted life-year QOL = quality of life RHC = right heart catheterization SC = subcutaneous TTCW = time to clinical worsening TTE = transthoracic echocardiogram WHO FC = World Health Organization functional class References 1. Taylor B, Rumbak M, Taylor SP, Solomon D. Early versus delayed right heart catheterization in evaluation of pulmonary arterial hypertension. J Heart Lung Transplant. 2013;32:137-138. 2. Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493-2537. 3. McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 suppl):14S-34S. 4. Burger CD, Long PK, Shah MR, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest. 2014;146:1263-1273. References (cont) 5. Johnson S, Delate T, Boka A, et al. Characterizing the financial burden of pulmonary arterial hypertension within an integrated healthcare delivery system. J Med Econ. 2013;16:1414-1422. 6. Galiè N. The AMBITION study: design and results. Presented at: 2014 European Respiratory Society Annual Meeting; June 9-14, 2014; Munich, Germany. Abstract 2916. 7. McGoon MD, Kane GC. Pulmonary hypertension: diagnosis and management. Mayo Clin Proc. 2009;84:191-207. 8. Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809-818. 9. Mehta S, Delcroix M, Galiè N, et al. Macitentan reduced all-cause hospitalizations in patients with pulmonary arterial hypertension: data from the randomized controlled SERAPHIN trial. Presented at: 2014 American Thoracic Society Annual Meeting; May 16-21, 2014; San Diego, CA. Abstract A2458. References (cont) 10. Actelion press release. Selexipag meets primary endpoint in pivotal phase III GRIPHON outcome study in patients with pulmonary arterial hypertension. http://www1.actelion.com/en/our-company/news-and-events.page?newsId=1793163. Accessed December 15, 2014. 11. McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250-2294. 12. Frumkin LR. The pharmacological treatment of pulmonary arterial hypertension. Pharmacol Rev. 2012;64(3):583-620. 13. Jing ZC, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation. 2013;127:624-633. References (cont) 14. White RJ, Chakinala MM, Mathier M, et al. Safety and tolerability of transitioning from parenteral treprostinil to oral treprostinil in patients with pulmonary arterial hypertension. Presented at: 2013 American Thoracic Society Annual Meeting; May 17-23, 2013; Philadelphia, PA. Abstract A3303. 15. Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330-340. 16. Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319-329. 17. Walker T. FDA approves first drug to treat two forms of pulmonary hypertension. Drug Topics. October 10, 2013. http://drugtopics.modernmedicine.com/drugtopics/content/clinical/clinical-pharmacology/fda-approves-first-drug-treat-two-formspulmonary?page=full. Accessed December 15, 2013.