Using Clinical Data for Scholarly Projects
advertisement
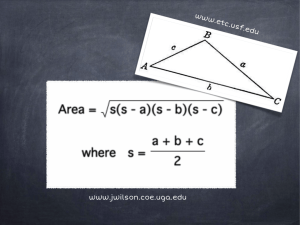
DATA Role of data in QI and Scholarship Sources/categories of data Characteristics of “good” data Administrative databases – pros &cons New Informatics support for Scholarship, QI, and Translational Research Data are the tools for quality improvement “Learning Healthcare System” Data Sources Clinical Data Review medical records Registries Clinical Trials Administrative Data Bases Proprietary UHC, Premier, HMO’s Government VAH, CMS Specialty organizations NIH funded Industry/FDA Industry registries CDC, States wwww.ClinicalTrials.gov Difference between Clinical Data and Administrative Data Bases • Clinical data (National Surgical Quality Improvement Program) – – – • – Prospective data collection, chart abstraction Expensive, labor-intensive Face validity among physicians Administrative data base (UHC’s CDB, Premier, ThomsonReuters) – Always retrospective, Claims data (medical record coding) – Can study resource use and cost of care – Very efficient way to collect data I2B2 – Integrating Informatics for Biology and Bedside – HERON (Healthcare Enterprise Repository Ontological Narration) at KUMC – Software program - integration of EPIC, Clinical information, IDX of retrospective data Where do the data elements come from? Physician: Documentation of patient care Coders: Assignment of codes to diagnoses and procedures Creation of a ‘CLAIM’ with patient demographics; DRG; diagnoses and procedures; LOS; charges; admission/discharge dates, status; physician; etc. Payers (e.g. CMS, BCBS) State UHC Clinical Data Base (CDB) Died Survived Risk Model Low Risk High Risk A robust model should assign higher probability of death to patients who died than to those who survived, at least 70% of the time (i.e. c-index >= 0.70) UHC Risk Adjustment Overview 2008 Potentially avoidable complications (not input into the model) Age Gender Race Socioeconomic status (Medicaid, self pay, charity, no charge) Admission status (emergency) Transfer status, acute hospital, nursing home Palliative care DRG-specific conditions Ventilator on Day 1 Severity-of-illness class for DRG based models risk of mortality Inputs Up to 30 comorbid or chronic conditions (e.g. diabetes, liver disease, obesity) Separate regression models for Cost, LOS, mortality for each DRG Expected mortality Expected cost Expected LOS What Variables Are Studied Almost anything having to do with an inpatient stay (ambulatory variables currently in development) Risk Adjusted Outcomes – Observed and Expected (O/E) for LOS, Mortality and Cost Complications, Readmissions, AHRQ Patient Safety Indicators Performance based on: Resource Utilization*: Hospitals Product Lines DRGs & MS-DRGs Diagnoses / Procedures Physicians Discharge Date/Month/Year Patient Demographics Blood Products Drugs Imaging Tests ICU Med/Surg Supplies Pharmacy * Resource Manager If you want to use UHC database? • Develop your proposal • Contact : Chris Wittkopp – Organizational improvement • Discuss your proposal and her assessment of data retrieval strengths • Write short proposal with background, purpose, methods • Submit proposal to Human Subject review • If QI project can get exemption Frontiers (CTSA)Biomedical Informatics Goals • Portal for investigators to access clinical and transitional research resources, track usage, and provide informatics consultative services • Create a platform, HERON, to integrate clinical and biological data for translational research • Link biological tissues to data generated by research cores • Leverage statewide telemedicine and Health Information Exchange (HIE) to support community based translational research What is HERON? HERON (Healthcare Enterprise Repository for Ontological Narration) is a search discovery tool that allows you to search deidentified data from various hospital and medical center sources that include but are not limited to Epic/O2 (the hospital electronic medical record), IDX (the clinical billing system), KU Hospital Cancer Registry, KU Biospecimen Repository, REDCap (selected projects), Social Security Death Index, and University HealthSystem Consortium (Quality Measure Data). By combining the various data sources, researchers can look at the data in new ways not available when viewing data in one source at a time. Why should I use HERON? HERON is a powerful tool that can save time during your research process. Searching across multiple data resources allows you to view data trends, key in on your research criteria, modify your search requirements and see how the data changes. This is a good tool to employ at the start a research project as it saves time by helping you focus and define your research. The HERON tool also provides analysis tools, such as the Timeline and the Cancer Survival Analysis tools. Larger projects where biostats sets up data sets, does monitoring and auditing, ie funded RCT Data management tool, each investigator enters and monitors own data Frontiers (CTSA)Biomedical Informatics Goals • 1) Portal for investigators to access clinical and transitional research resources, track usage, and provide informatics consultative services • 2) Create a platform, HERON, to integrate clinical and biological data for translational research • 3) Link biological tissues to data generated by research cores • 4) Leverage statewide telemedicine and Health Information Exchange (HIE) to support community based translational research Summary • Data is essential for Scholarship, Quality Improvement and Education • Sources of data are multiple • • • • Clinical Administrative Registry Informatics for Integrating Biology with Bedside – HERON – Data Management Systems – CRIS – RedCap