Medications - e
advertisement

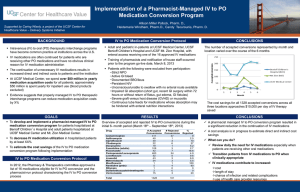
Dr. Helen Chen1, Kevin Quach1,2, Dr. Ian MacKillop1, Dr. Joseph Kim2 1 University of Waterloo 2 University Health Network Faculty: Dr. Helen Chen, PhD Relationships with commercial interests: None 1. 2. 3. Challenges of patient drug management in transplantation Drug standardization strategies Lessons learned and continuous discovery Standard for safe medication practices Five rights: Patient, drug, time, dose, and route Patient safety Incomplete information about patient’s medications Knowledge deficit leading to administration of wrong dose or use of wrong route Serious drug interaction unknown or overlooked Source: Medication Errors, Cohen, 2006 Need to precisely manage patient medications in the continuum of care Lack of standard drug nomenclature Lack of information management process Interoperability of multiple drug information systems Fast evolving of drug products and variations of nomenclature Recognized and took action to resolve a medication information problem Established a working committee (physicians, nurses, pharmacists, IT specialists, IT admin) Consulted with standardized drug databases (e.g. Health Canada, SNOMED, RxNorm) Established medication standardization strategies Developed a data governance policy for future maintenance of drug database 1. Removing non-prescribed drugs from drug database 2. Correcting misspelled drug names 3. Mapping drug name with drug formula and strength 4. Removing unnecessary use of chemical salts 5. Distinguishing between brand name and generic name medications 6. Correcting combination drug nomenclature 7. Adhering to Health Canada drug nomenclature standards 8. Identifying drug acronyms and other variations 9. Proper labeling study medications 10. Identifying limited use medications Medication name Medication name Venlaxafine venlaxafine Venlaxafine Cap XR 150 mg Venlaxafine Cap XR 75 mg Venlaxafine Cap XR 37.5 mg Venlaxafine Tab 37.5 mg Venlaxafine Tab 75mg Brand name Effexor XR Effexor Formulation 37.5 mg capsule 37.5 mg tablet Solution Drug formula and dosage information will be retained in separate tables from medication name Medication name Tylenol Generic name acetaminophen Brand name Tylenol Prograf tacrolimus Prograf Solution Merging brand name information to the corresponding generic name Medication Name hydroxyephedrine w/ normethadone Proposed Correction hydroxyephedrine - normethadone Piperacillin, tazobactam piperacillin - tazobactam Other problems Specifying doses of each drug ingredient in combination drugs Solution Using a dash (-) to separate drug ingredients in combination medications Medication Name Prograf – LU #173 Proposed Correction Prograf Valacyclovir LU #159 Valacyclovir Problem • Limited use medications are offered under the Ontario Drug Benefit Program for specific situations as for a particular medical condition and/or for a limited period of time (Source: Ontario Ministry of Health and Long-Term Care) • Physicians are required to review the criteria before writing the three-digit code Solution • Removing LU codes from medication names • Implementing an alert system to notify physicians when LU medications are being added to patient chart VID list List of medications to import all updated brand name medication information Medications included ▪ Frequently recorded medications (e.g. acetaminophen) ▪ Immunosuppressants (e.g. cyclosporine, MMF) Synchronization tool with Health Canada All medication entries (n=3189) Non-prescribed entries (n=1112) Prescribed entries (n=2077) Strategy Action identified Mapping drug name with drug formula and strength 643 (31%) Distinguishing between brand name and generic name medications 228 (11%) Correcting misspelled drug names 145 (7%) Removing unnecessary use of chemical salts 124 (6%) Correcting combination drug nomenclature 81 (4%) Sorting study medications 103 (5%) Limited use medications 18 (0.7%) Other 91 (4.3%) No change needed 644 (31%) Total 2077 Aforementioned challenges continue to exist Solutions Understand importance of drug nomenclature standardization and adhere to strategies Develop robust processes to regulate changes Construct technological tools to perform updates