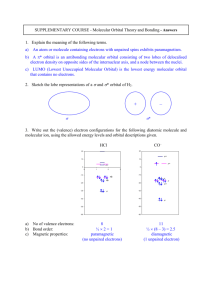
Classification of the Elements
advertisement

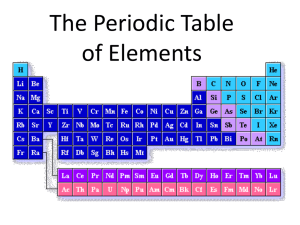
Classification of the Elements Objectives: AHSGE Reading 4.3 Discern organizational patterns. Other AOD C.3.2 Recognize periodic trends of elements, including the number of valence electrons, atomic size, and reactivity. Organizing the Elements by Electron Configuration • We know valence electron configurations of the group A elements. Now let’s look at valence electrons and periods. • Write the noble gas configurations of the group 1A elements straight down in a column. • What is the 1st trend you notice? • What other trend do you see? Organizing the Elements by Electron Configuration (continued) • The energy level of the valence electrons is the same as the period number the element is found in! • So in what energy level would the valence electrons for gallium (Ga) be found? • What about titanium (Ti)? s-, p-, d-, and f- Block Elements • s-block elements have valence electrons in the s orbital. • What groups would this be? • p-block elements are found in groups 3A8A. • This block’s elements have filled or partially filled p orbitals. • How many groups on the periodic table are in the p-block? Why is this so? s-, p-, d-, and f- Block Elements (continued) • The first period contains NO p-block elements. The first p-block element is boron. • What period is boron in? • Why are there no p-block elements in the first period? Answer: The 1st period elements only have a 1s orbital. s-, p-, d-, and f- Block Elements (continued) • d-block elements are the transition metals. • USUALLY contain a filled outermost sorbital of energy level n, and a filled or partially filled d-orbital of energy level n-1. • Example: Scandium (Sc) is in period ___. • Sc has a filled 4s orbital and a partially filled 3d orbital. s-, p-, d-, and f- Block Elements (continued) • So what s-orbital and what p-orbital would be filled or partially filled for tungsten (W)? Answer: 6s and 5p. • How many d orbitals are there? Answer: 5 • How many electrons can those d orbitals hold? Answer: 10 s-, p-, d-, and f- Block Elements (continued) • How many “groups” (columns) does the d-block span on the periodic table? • f-block elements are the inner transition metals. • Characterized by a filled or partially filled outermost s-orbital, and filled or partially filled 4f or 5f orbital. • f-orbitals do not fill in a predictable manner. Overview/Review 1. Moving DOWN through the periods, the principal energy level increases. 2. As principal energy level increases, so does the number of energy sublevels containing electrons. 3. Period 1 has only s-block elements. 4. Periods 2 and 3 have s- and p-orbitals. 5. Periods 4 and 5 have s-, p- and dorbitals. 6. Periods 6 and 7 have ?????? Assignments 1. Practice Problems, p.162 (7-9) 2. In which block of the periodic table would each of the elements identified in #9 be found? 3. Complete Study Guide for Content Mastery, pp.33-34 (16-26).