Density
advertisement

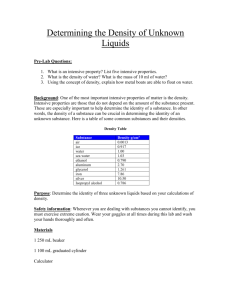
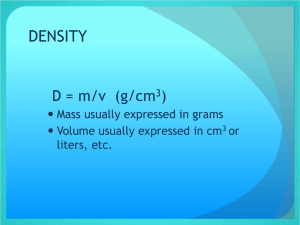
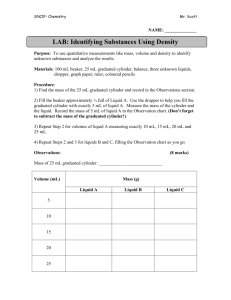
Density A physical property Density Density - the amount of mass per unit of volume. It can be expressed in the following formula. Density The formula… Density = mass volume Or D=m/v Understanding Density In order to understand density, you must first understand mass and volume. Mass-The amount of matter in an object. (balance/scale) Volume-The amount of space occupied by an object. (ruler and formula or graduated cylinder) So density is just the amount of matter in an object divided by the space that the object occupies. Density Density = mass / volume Density compares mass to volume. If you have any two components, you can “figure out” mathematically the third component by substituting the numbers in the formula. (D=m/v) Density Density = mass / volume To calculate density, substitute numbers (preferable from your data) for mass and volume. You would use a scale or a balance to find mass, and you could either use a formula (l x w x h) or use a graduated cylinder to find volume. Volume The formula for volume is … V=lxwxh (volume = length x width x height) Or, you could simply see how much water a solid or liquid object displaces in a graduated cylinder. Density You can even determine the relative densities of liquids by the layers that are formed when they are mixed, or what floats upon what. More dense substances sink. Less dense substances float. Density of Water The density of water is 1gram per milliliter, or D=1g/ml Substances that are more dense than 1g/ml will sink when placed in water. Substances that are less dense than 1g/ml will float when placed in water. Density in Solids A couple of video clips might help with your understanding… D = m/v……………..where v = l x w x h Practical applications of density Density in Liquids A couple of video clips might help with your understanding… When liquids don’t mix Density of water, sinkers and floaters