P1 1.7 Specific heat capacities
advertisement

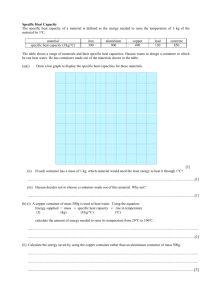
So taking a bite….this happens…..which ingredient has caused the problem and why? P1 1.7 Specific heat capacities. Learning Objectives • Explain what specific heat capacity is. • Use the equation for specific heat capacity. • Evaluate the use of materials according to their specific heat capacities. • If you give the same amount of heat to different type of matters you observe that changes in their temperatures are different. • Given an equal amount of heat to metal spoons and wooden spoons, metal spoon has greater change in its temperature. • These examples show that each matter has its own characteristics to absorb heat. • We call this concept as specific heat capacity of the matters. • It is the distinguishing property of matters. • We show it with the letter “c” and give the definition of it as, heat required to increase temperature of unit mass 1 ºC. • On the contrary, heat capacity of the system is defined as “heat required increasing the temperature of whole substance” and we show it with “C”. • E=m.c where m is the mass of the substance and c is the specific heat of the matter. The specific heat capacity equation. • E = m cΔT Where • E is the energy in Joules • m is the mass of the material in kg • c is the specific heat capacity of the material (this will be given to you) • Δ T is the change in temperature °C Exam feedback ….in an exam you will be expected to be able to re-arrange equations and put the quantity you need to calculate on the left. How much energy does a kettle transfer when it heats up 1 kg of water from 10˚c to100 ˚c? The specific heat capacity of water is 4200J/kg ˚c E = m x c x Heat mass specific heat temp change Transferred capacity 378kJ = 1kg x 4200 x 90 Questions 1. A student heats 1kg block of aluminium and a 1kg block of copper to 50 °C. Explain which block will be storing the most energy. 2. Why does it take longer to boil a kettle full of water than one only half full? 3. Explain why stand- alone radiators are filled with oil, instead of being filled with air? Questions 4. How much energy does it take to heat up 500g of lead by 30 °C ? 5. A washing machine heats 10kg of water for each wash cycle. How much energy is saved by washing clothes at 30 °C instead of 50 °C ? 6. A storage heater contains 100kg of concrete. 1800kJ of energy is transferred to it. What is the temperature change of the concrete? The specific heat capacities of some materials. Material Specific heat capacity J/kg °C Air 100 Aluminium 899 Concrete 900 Copper 390 Iron 450 Lead 130 Oil 540 Water 4200