Nucleation - Clarkson University
advertisement

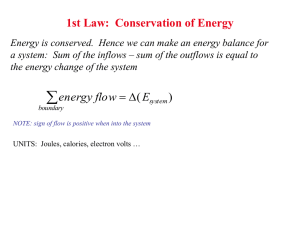
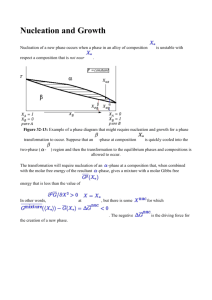
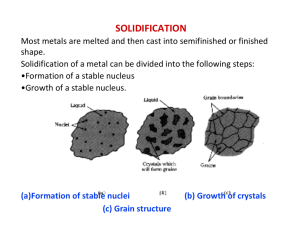
Nucleation Don H. Rasmussen Box 5705 Clarkson University rasmu@clarkson.edu Homogeneous Nucleation Fluctuations in composition and structure which are small in extent but large in degree result in small new phase nuclei which are in local equilibrium but unstable to growth in an undercooled or supersaturated parent phase. How does Homogeneous Nucleation Occur? Stable clusters form when their formation decreases total free energy. Growth of small clusters is limited because new particle surface costs more free energy than the bulk free energy reduction. Only large clusters are stable. Clusters grow and decay by monomer addition/evaporation and there is in a metastable cluster size distribution. The larger the supersaturation or undercooling, the greater the number and maximum size of the existing clusters and the smaller the necessary critical cluster size for continued growth. For clusters just larger than critical, the growth rate increases along with the size in an autocatalytic fashion. Numerical Model of Homogeneous Nucleation Nucleation Rate, J, is the product of q, the net probability of an atom jumping across interface and into the critical cluster (per unit surface area) t, jump frequency of monomer is fluid Ac, the surface area of the critical cluster nc, the concentration of critical clusters per unit volume J q t Ac nc q is the net rate of diffusion across the surface of a cluster dC ci co q D D dx l where Do is the diffusion coefficient in the liquid. Qd D Do exp RT and ci and co are the concentration of crystallizing atoms on the two sides of the interface of thickness, l. The surface area of a spherical cluster is Ac 4 r 2 The concentration of critical clusters is G f nc no exp RT Nucleation Rate per Unit Volume, J G f (co ci ) Qd J qt Acnc no t Ac Do exp exp kT l kT The pre-exponential factors are almost constant and approximately 1035 nuclei/cm3sec. G f nuclei Qd J 10 exp exp 3 kT kT cm sec 35 Effect of Temperature on Bulk Free Energy Change Free energy T Gv H TS H 1 TM Gv L S GS GL T T TE Temperature Free Energy of a Cluster as a Function of Size 4 G f (r ) 4 r r 3 Gv 3 2 9 2 10 9 2 10 9 1 10 dA( r ) F ( r ) 0 VdP ( r ) 9 1 10 9 2 10 2 109 0 0 20 40 60 r 80 100 100 Influence of increasing Undercooling or Supersaturation From 1 to 5 the supersaturation or undercooling increases which results in a decrease in both the critical cluster size and the barrier to nucleation. 9 1 10 F 1 ( r ) 8 5 10 0 F 2 ( r ) F 3 ( r ) F 4 ( r ) F 5 ( r ) 8 5 10 9 1 10 9 1.5 10 9 2 10 0 20 40 60 r 80 100 Conditions for critical cluster or nucleus d [G f (r )] dr 0 8 r 4 r 2 Gv 2 r Gv * 16 16 T G 2 3 Gv 3 H 2 T 2 3 * 3 2 M Critical Cluster Size and Free Energy Barrier versus Undercooling Free Energy Barrier Radius (nm) 100 50 0 0 50 Undercooling 100 5 1 10 4 5 10 0 0 50 Undercooling 100 Temperature Dependence of Nucleation Rate G f nuclei Qd J 10 exp exp 3 kT kT cm sec 35 33 1 10 J(T) 32 1 10 31 1 10 30 1 10 0 100 T 200 Time Temperature Transformation Curves The delay time is related to the reciprocal of the nucleation rate and here the delay time is plotted as a function of the undercooling. 0 T 200 1 10 100 Time (Seconds) 3 1 10 Heterogeneous Nucleation Nucleation at the surface of an impurity particle or on the walls of the container. “Catalysts for Nucleation” are surfaces which significantly lower the barrier to new phase formation. Heterogeneous nucleation occurs at low undercooling and at high rates. Nucleation on a substrate takes less material liquid nl sl q nucleus sn substrate Fraction of the critical cluster which must form at any specific undercooling Exposed Volume 1 2 f (q ) 2 cos(q ) 1 cos(q ) 4 1 0.5 0 0 2 4 Contact Angle (radians) Free Energy of Formation of the Nucleus versus Contact Angle at Fixed Undercooling 3 16 1 * 2 G (2 cos(q ) )(1 cos(q )) 2 3 Gv 4 Barrier Height 5 1.5 10 5 1 10 4 5 10 0 0 1 2 3 Contact An gle