Reading Worksheet Class 13 Physics 585 Please complete & bring
advertisement

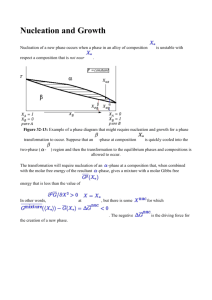
Reading Worksheet Class 14 Physics 585 Please complete & bring to class on Thursday. We will use it to guide discussion and you will hand it in later. Name______________ Your QUESTIONS are important. WRITE down at least two you have. Also put star by the items of greatest misunderstanding. Class 14: Ohring Chapter 7.4 through end of 7, pages 376-410. Look at and understand all of the figures in chapter. 7.3. Thermodynamic Aspects of Nucleation 376-86 You are now going to meet capillary theory. This is very useful. 1. Please study figures 7-11 and 7-12. 2. What is the paradox mentioned on 376-77? Look at the questions at top of 377. Surface Energies 3. What units are used? 4. Top of p 378 study what is relationship between surface energy and surface tension. Capillary theory 5. Study hetero vs. homo nucleation. 6. Can you identify all of the terms in eqn. 7-6? (Keep reading.) 7. If r is very small what is sign of ΔG? 8. If r is very large what is sign of ΔG? 9. What does the volume term do to the sign of ΔG? 10. How do they relate to fig 7-11? 11. What does the * mean? (below eqn 7-7 on page 379) (Keep reading.) 12. Eqn. 7-10 has two factors. Which corresponds to homogeneous nucleation ΔG? 13. What term in 7-10 is altered if there is a screw dislocation? How does ΔG* change? 14. Read last paragraph on bottom of page 380. 7.3.4 How do the 3 growth modes fall out of the analysis? How do they relate to atomic models? 15. New symbol at top of p 382 misfit. What is it? 16. What does it mean? 17. What is the factor that causes two-dimensional growth to switch to 3D? Study figure 7-12. 7.3.5 A study of number of nuclei that form at different temperatures & deposition rates. 18. Look at figure 7-13. Try to understand what it is saying before you read the text. 19. The eqns. 7-20 to 7-24 involve 4 unknowns. What are they? 20. The partial derivatives can be used to establish trends. See fig. 7-13. 21. T/F Monocrystals are preferred by many nuclei and high temperatures. 22. The bottom of 385 and top of 386 have the take home messages. Continuum models predict nuclei of only a few atoms. Do we switch to atomic (also called, microscopic) models? (See below.) 7.4 Kinetic processes in Nucleation and growth. Kinetics is about how a process depends on time. Here the processes are the formation of nuclei and their growth before a continuous film is formed. Here are the questions that 7.4.2 Nucleation Rate 23. Can atoms that strike a surface and condense reevaporate? (eqn. 7-25) 24. What is a typical value of vibration frequency? 25. Which quantity changes once an atom is absorbed that keeps it from evaporating? 26. Where does the supersaturation enter into eqn. 7-33? 7.4.3 Atomistic Models of the Nucleation Rate 27. What does i* stand for in 7-37? 28. Be ready to explain figure 7-14. Where would one expect to get the largest crystals? 29. What is E2 in equation 7-38? 30. Figure 7-15 is your friend. Study it. 31. To achieve epitaxy high surface diffusion constants are required. But how high? 32. What are the epitaxial temperatures for semiconductors, metals and alkali halides? 7.4.4 Kinetic Models of Nucleation Looking at the formation of nuclei as a chemical reaction to get the NUMBER of clusters of each size. 33. Do you understand all of the terms in 7-39 & 7-40? 34. Why do the curves in figure 7-16 show a decrease with long times? 35. How does the number of clusters depend on temperature? 36. What reason can one give for this? 37. Look at the Venables’ equation and Table 7-1 7.4.5 Cluster Coalescence & Depletion 38. Look at the features on page 394. 39. Look at figure 7-17. What is the difference between Oswald ripening and sintering? 40. What is the driving “force” that pushes ripening? 41. Look at figure 7-18. 42. Equation 7-43 tells about the kinetics/ mechanism of sintering. Based on values of n & m what is the mechanism of island sintering in thin films? (p 397) Cluster migration. Really? The whole thing migrates like a cattle herd? Coalescence and grain size: Metallurgy? 43. What is the Avrami equation? Is it about kinetics or thermodynamics? 44. How does grain size depend on linear growth rate? How about nucleation rate? 7.5 Experimental studies of Nucleation & Growth. 7.5.1 Electron Microscopy 45. TEM with postdeposition carbon overlay. What are the advantages/ disadvantages? 46. How can in situ studies be done? 7.5.2 AES: Auger Electron Spectroscopy: Hint: Auger is pronounced. Oh zshay 47. Study figure 7-19. 7.5.3 Metal film results. The most studied have been noble metals on ionic substrates. 48. Please give an example of such a pair. 49. Note three reasons why high substrate temperatures have been used. 7.5.4 Scanning Probe Microscopy Si on (001) Si 50. Can you help us understand what the three terraces in figure 7-20 are? 51. What is the activation energy in figure 7-21 due to? 52. How does it compare to the strength of a Si-bond? (Ask me in class) Metals on Si & Metals on Metals & WxN on 53. Study fig 7-22 & 7-23 54. Are WxN grains beach balls or pancakes? 7.6 Conclusion: What questions do you have?