Ch. 16
advertisement

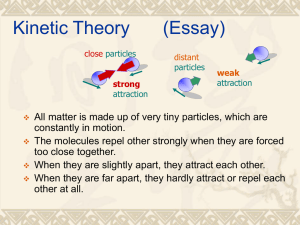
Chapter 16 Thermal Properties of Matter Macroscopic Description of Matter State Variables • State variable = macroscopic property of thermodynamic system • Examples: pressure p volume V temperature T mass m State Variables • State variables: p, V, T, m • I general, we cannot change one variable without affecting a change in the others • Recall: For a gas, we defined temperature T (in kelvins) using the gas pressure p Equation of State • State variables: p, V, T, m • The relationship among these: ‘equation of state’ • sometimes: an algebraic equation exists • often: just numerical data Equation of State • • • • Warm-up example: Approximate equation of state for a solid Based on concepts we already developed Here: state variables are p, V, T V 1 1 (T T0 ) ( p p0 ) V0 B Derive the equation of state The ‘Ideal’ Gas • The state variables of a gas are easy to study: • p, V, T, mgas • often use: n = number of ‘moles’ instead of mgas Moles and Avogadro’s Number NA • 1 mole = 1 mol = 6.02×1023 molecules = NA molecules • n = number of moles of gas • M = mass of 1 mole of gas • mgas = n M Do Exercise 16-53 The ‘Ideal’ Gas • We measure: the state variables (p, V, T, n) for many different gases • We find: at low density, all gases obey the same equation of state! Ideal Gas Equation of State • State variables: p, V, T, n pV = nRT • p = absolute pressure (not gauge pressure!) • T = absolute temperature (in kelvins!) • n = number of moles of gas Ideal Gas Equation of State • State variables: p, V, T, n pV = nRT • R = 8.3145 J/(mol·K) • same value of R for all (low density) gases • same (simple, ‘ideal’) equation Do Exercises 16-9, 16-12 Ideal Gas Equation of State • State variables: p, V, T, and mgas= nM pV mgas M RT • State variables: p, V, T, and r = mgas/V M p r R T Derive ‘Law of Atmospheres’ Non-Ideal Gases? • Ideal gas equation: pV nRT • Van der Waals equation: n2 p a 2 (V bn) nRT V Notes pV–Diagram for an Ideal Gas Notes pV–Diagram for a Non-Ideal Gas Notes Microscopic Description of Matter Ideal Gas Equation pV = nRT • n = number of moles of gas = N/NA • R = 8.3145 J/(mol·K) • N = number of molecules of gas • NA = 6.02×1023 molecules/mol Ideal Gas Equation pV nRT N RT NA NkT • k = Boltzmann constant = R/NA = 1.381×10-23 J/(molecule·K) Ideal Gas Equation pV = nRT pV = NkT • k = R/NA • ‘ RT per mol’ vs. ‘kT per molecule’ Kinetic-Molecular Theory of an Ideal Gas Assumptions • gas = large number N of identical molecules • molecule = point particle, mass m • molecules collide with container walls = origin of macroscopic pressure of gas Kinetic Model • molecules collide with container walls • assume perfectly elastic collisions • walls are infinitely massive (no recoil) Elastic Collision • wall: infinitely massive, doesn’t recoil • molecule: vy: unchanged vx : reverses direction speed v : unchanged Kinetic Model • For one molecule: v2 = vx2 + vy2 + vz2 • Each molecule has a different speed • Consider averaging over all molecules Kinetic Model • average over all molecules: (v2)av= (vx2 + vy2 + vz2)av = (vx2)av+(vy2)av+(vz2)av = 3 (vx2)av Kinetic Model • (Ktr)av= total kinetic energy of gas due to translation • Derive result: 2 pV ( K tr ) av 3 Kinetic Model 2 pV ( K tr ) av 3 • Compare to ideal gas law: pV = nRT pV = NkT Kinetic Energy 3 ( K tr ) av nRT 2 3 NkT 2 • average translational KE is directly proportional to gas temperature T Kinetic Energy • average translational KE per molecule: 1 3 2 m(v ) av kT 2 2 • average translational KE per mole: 1 3 2 M (v ) av RT 2 2 Kinetic Energy • average translational KE per molecule: 1 3 2 m(v ) av kT 2 2 • independent of p, V, and kind of molecule • for same T, all molecules (any m) have the same average translational KE Kinetic Model 3kT 3RT (v ) av m M 2 • ‘root-mean-square’ speed vrms: vrms 3kT 3RT (v ) av m M 2 Molecular Speeds vrms 3kT 3RT (v ) av m M 2 • For a given T, lighter molecules move faster • Explains why Earth’s atmosphere contains alomost no hydrogen, only heavier gases Molecular Speeds • Each molecule has a different speed, v • We averaged over all molecules • Can calculate the speed distribution, f(v) (but we’ll just quote the result) Molecular Speeds f(v) = distribution function f(v) dv = probability a molecule has speed between v and v+dv dN = number of molecules with speed between v and v+dv = N f(v) dv Molecular Speeds • Maxwell-Boltzmann distribution function m f (v) 4 2kT 3/ 2 2 v 2e mv / 2kT Molecular Speeds • At higher T: more molecules have higher speeds • Area under f(v) = fraction of molecules with speeds in range: v1 < v < v1 or v > vA Molecular Speeds • average speed vav 0 8kT v f (v) dv m • rms speed vrms (v 2 ) av (v ) av 2 0 3kT v f (v) dv m 2 Molecular Collisions? • We assumed: • molecules = point particles, no collisions • Real gas molecules: • have finite size and collide • Find ‘mean free path’ between collisions Molecular Collisions Molecular Collisions • Mean free path between collisions: 1 4 2 r 2 ( N / V ) kT 4 2 r 2 p Announcements • • • • Midterms: Returned at end of class Scores will be entered on classweb soon Solutions available online at E-Res soon • Homework 7 (Ch. 16): on webpage • Homework 8 (Ch. 17): to appear soon Heat Capacity Revisited Heat Capacity Revisited DQ mc DT DQ = energy required to change temperature of mass m by DT c = ‘specific heat capacity’ = energy required per (unit mass × unit DT) Heat Capacity Revisited DQ mc DT • Now introduce ‘molar heat capacity’ C C = energy per (mol × unit DT) required to change temperature of n moles by DT DQ nC DT Heat Capacity Revisited DQ nC DT • important case: the volume V of material is held constant • CV = molar heat capacity at constant volume DQ nCV DT CV for the Ideal Gas • Monatomic gas: • molecules = pointlike (studied last lecture) • recall: translational KE of gas averaged over all molecules (Ktr)av = (3/2) nRT CV for the Ideal Gas • Monatomic gas: (Ktr)av = (3/2) nRT • note: your text just writes Ktr instead of (Ktr)av • Consider changing T by dT CV for the Ideal Gas • Monatomic gas: (Ktr)av = (3/2) nRT d(Ktr)av = n (3/2)R dT • recall: dQ = n CV dT • so identify: CV = (3/2)R In General: If Then But recall: So we identify: (Etot)av = (f/2) nRT d(Etot)av = n (f/2)R dT dQ = n CV dT CV = (f/2)R A Look Ahead (Etot)av = (f/2) nRT CV = (f/2)R Monatomic gas: Diatomic gas: f=3 f = 3, 5, 7 CV for the Ideal Gas • What about gases with other kinds of molecules? • diatomic, triatomic, etc. • These molecules are not pointlike CV for the Ideal Gas • Diatomic gas: • molecules = ‘dumbell’ shape • its energy takes several forms: (a) translational KE (3 directions) (b) rotational KE (2 rotation axes) (c) vibrational KE and PE Demonstration CV for the Ideal Gas • Diatomic gas: Etot = Ktr + Krot + Evib (Etot)av = (Ktr)av + (Krot)av + (Evib)av • we know: (Ktr)av = (3/2) nRT • what about the other terms? Equipartition of Energy • Can be proved, but we’ll just use the result • Define: f = number of degrees of freedom = number of independent ways that a molecule can store energy Equipartition of Energy • It can be shown: • The average amount of energy in each degree of freedom is: (1/2) kT per molecule i.e. (1/2) RT per mole Check a known case • Monatomic gas: • only has translational KE in 3 directions: vx, vy, vz • f = 3 degrees of freedom (Ktr)av = (f/2) nRT = (3/2) nRT CV for the Ideal Gas • Diatomic gas: • more forms of energy are available to the gas as you increase its T: (a) translational KE (3 directions) (b) rotational KE (2 rotation axes) (c) vibrational KE and PE A Look Ahead (Etot)av = (f/2) nRT CV = (f/2)R Monatomic gas: Diatomic gas: f=3 f = 3, 5, 7 CV for the Ideal Gas • Diatomic gas: low temperature • only translational KE in 3 directions: vx, vy, vz • f = 3 degrees of freedom (Etot)av = (f/2) nRT = (3/2) nRT CV for the Ideal Gas • Diatomic gas: higher temperature • translational KE (in 3 directions) • rotational KE (about 2 axes) • f = 3+2 = 5 degrees of freedom (Etot)av = (f/2) nRT = (5/2) nRT CV for the Ideal Gas • Diatomic gas: even higher temperature • translational KE (in 3 directions) • rotational KE (about 2 axes) • vibrational KE and PE • f = 3+2+2 =7 degrees of freedom (Etot)av = (f/2) nRT = (7/2) nRT Summary of CV for Ideal Gases (Etot)av = (f/2) nRT CV = (f/2)R Monatomic: Diatomic: f = 3 (only) f = 3, 5, 7 (with increasing T) CV for Solids • Each atom in a solid can vibrate about its equilibrium position • Atoms undergo simple harmonic motion in all 3 directions CV for Solids • Kinetic energy : 3 degrees of freedom • K = Kx+ Ky + Kz • Kx = (1/2) mvx2 • Ky = (1/2) mvy2 • Kz = (1/2) mvz2 CV for Solids • Potential energy: 3 degrees of freedom • U = Ux+ Uy + Uz • Ux = (1/2) kx x2 • Uy = (1/2) ky y2 • Uz = (1/2) kz z2 CV for Solids • f=3+3=6 degrees of freedom (Etot)av = (f/2) nRT = 3 nRT CV = (f/2)R = 3 R Phase Changes Revisited Phase Changes • ‘phase’ = state of matter = solid, liquid, vapor • during a phase transition : 2 phases coexist • at the triple point : all 3 phases coexist Do Exercise 16-39 pT Phase Diagram pV–Diagram for a Non-Ideal Gas Notes Announcements • • • • Midterms: Returned at end of class Scores will be entered on classweb soon Solutions available online at E-Res soon • Homework 7 (Ch. 16): on webpage • Homework 8 (Ch. 17): to appear soon