CH121a Atomic Level Simulations of Materials and
Molecules
Instructor: William A. Goddard III
Prerequisites: some knowledge of quantum mechanics, classical
mechanics, thermodynamics, chemistry, Unix. At least at the Ch2a
level
Ch121a is meant to be a practical hands-on introduction to
expose students to the tools of modern computational
chemistry and computational materials science relevant to
atomistic descriptions of the structures and properties of
chemical, biological, and materials systems.
This course is aimed at experimentalists (and theorists) in
chemistry, materials science, chemical engineering, applied
physics, biochemistry, physics, geophysics, and mechanical
engineering with an interest in characterizing and designing
molecules, drugs, and materials.
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
1
Motivation: Design Materials, Catalysts, Pharma from 1st Principles so
can do design prior to experiment
To connect 1st Principles (QM) to Macro work use an overlapping hierarchy of
methods (paradigms) (fine scale to coarse) so that parameters of coarse level
are determined by fine scale calculations.
Thus all simulations are first-principles based
time
ELECTRONS ATOMS
GRAINS
GRIDS
hours
Continuum
(FEM)
millisec
Micromechanical modeling
Protein clusters
MESO
nanosec
MD
picosec
Deformation and Failure
Protein Structure and Function
QM
femtosec
simulations real devices
full cell (systems biology)
distance
Å
nm
micron
mm
Big breakthrough making FC simulations
yards practical:
Accurate calculations for bulk phases
reactive force fields based on QM
and molecules (EOS, bond dissociation)
Describes: chemistry,charge transfer, etc. For
Lecture
1Ch121a-Goddard-L01
©
copyright
2011
William
A. Goddard
all rights reserved\
2
Chemical Reactions (P-450 oxidation)
metals,
oxides,III,organics.
Lectures
The lectures cover the basics of the fundamental methods:
quantum mechanics,
force fields,
molecular dynamics,
Monte Carlo,
statistical mechanics, etc.
required to understand the theoretical basis for the simulations
the homework applies these principles to practical problems
making use of modern generally available software.
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
3
Homework
First 6 weeks: The homework each week uses generally available
computer software implementing the basic methods on
applications aimed at exposing the students to understanding how
to use atomistic simulations to solve problems.
Each calculation requires making decisions on the specific
approaches and parameters relevant and how to analyze the
results.
Midterm: each student submits proposal for a project using the
methods of Ch120a to solve a research problem that can be
completed in 4 weeks.
The homework for the last 3 weeks is to turn in a one page report
on progress with the project
The final is a research report describing the calculations and
conclusions.
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
4
Methods to be covered in the lectures include:
Quantum Mechanics: Hartree Fock and Density Function
methods
Force Fields standard FF commonly used for simulations of
organic, biological, inorganic, metallic systems, reactions;
ReaxFF reactive force field: for describing chemical reactions,
shock decomposition, synthesis of films and nanotubes, catalysis
Molecular Dynamics: structure optimization, vibrations, phonons,
elastic moduli, Verlet, microcanonical, Nose, Gibbs
Monte Carlo and Statistical thermodynamics Growth
amorphous structures, Kubo relations, correlation functions, RIS,
CCBB, FH methods growth chains, Gauss coil, theta temp
Coarse grain approaches
eFF for electron dynamics
Tight Binding for electronic properties
solvation, diffusion,
mesoscale
force fields © copyright 2011 William A. Goddard III, all rights reserved\
Lecture 1Ch121a-Goddard-L01
5
Applications will include prototype examples
involving such materials as:
Organic molecules (structures, reactions);
Semiconductors (IV, III-V, surface reconstruction)
Ceramics (BaTiO3, LaSrCuOx)
Metal alloys (crystalline, amorphous, plasticity)
Polymers (amorphous, crystalline, RIS theory, block);
Protein structure, ligand docking
DNA-structure, ligand docking
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
6
Outline
Topic 1: QM: HF, DFT, basis sets, reactions, transition states, vibrations
Topic 2: Force Fields, nonbonds, hydrogen bonds, charges (QEq), QM FF
Topic 3: Molecular Dynamics: Verlet, NVE, NVT, NPT, Periodic Systems
Topic 4: Statistical mechanics: liquid simulations, entropy, nonequilibrium MD,
Green-Kubo, Monte Carlo, Grand Canonical MC, gas storage, surface tension
Topic 5: polymers: crystalline, amorphous, structure prediction,
Topic 6: ReaxFF Reactive Force Field and reactive Dynamics
Topic 7: PBC QM, band structure, phonons, elastic constants, Ab Initio MD,
Topic 8: surfaces: reconstruction, chemisorption, physisorption, solvation
Topic 9: eFF, Tight binding
Topic 9: applications:
Fuel Cell: oxygen reduction reaction, migration in Nafion solid oxides
Batteries: anode for Li battery, Solid electrolyte interface,
Nanotechnology: rotaxane molecular switches, carbon nanotube interfaces
Water Treatment: Dendrimers, captymers
Catalysis: alkane activation, ammoxidation
Metallic alloys: force fields, dislocations, plasticity, cavitation
Proteins: structures, ligand docking, GPCRs
DNA: A to B transition, Origami based nanotechnology
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
7
Topic 1: Practical Quantum Chemistry
Solve Schrödinger Equation HelΨ=EΨ
1 1 22
1Z A2Z B Z A Z B Z A
ZA 1
1
H
=
el Ai i
2M
i 2
A
i B2
A
RAB A B Ri ABA RAii
R r
A i jAi ij i j
rij
For benzene we have 12 nuclear
degrees of freedom (dof) and 42
electronic dof
For each set of nuclear dof, we solve HelΨ=EΨ to
calculate Ψ, the probability amplitudes for finding the 42
electrons at various locations
1st term: kinetic energy operator HOW MANY
2nd term: attraction of electrons to nuclei: HOW MANY
3rd term: electron-electron repulsion HOW MANY
th term:
1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
8
4Lecture
what is missing?
The Schrödinger Equation: Kinetic Energy
Solve Schrödinger Equation HelΨ=EΨ
1 1 22
1Z A2Z B Z A Z B Z A
ZA 1
1
H
=
el Ai i
2M
i 2
A
i B2
A
RAB A B Ri ABA RAii
R r
A i jAi ij i j
rij
For benzene we have 12 nuclear
degrees of freedom (dof) and 42
electronic dof
For each set of nuclear dof, we solve HelΨ=EΨ to
calculate Ψ, the probability amplitudes for finding the 42
electrons at various locations
1st term: kinetic energy operator: 42 terms
2nd term: attraction of electrons to nuclei: HOW MANY
3rd term: electron-electron repulsion HOW MANY
th term:
1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
9
4Lecture
what is missing?
The Schrödinger Equation: Nuclear-Electron
Solve Schrödinger Equation HelΨ=EΨ
1 1 22
1Z A2Z B Z A Z B Z A
ZA 1
1
H
=
el Ai i
2M
i 2
A
i B2
A
RAB A B Ri ABA RAii
R r
A i jAi ij i j
rij
For benzene we have 12 nuclear
degrees of freedom (dof) and 42
electronic dof
For each set of nuclear dof, we solve HelΨ=EΨ to
calculate Ψ, the probability amplitudes for finding the 42
electrons at various locations
1st term: kinetic energy operator: 42 terms
2nd term: attraction of electrons to nuclei: 42*12= 504
3rd term: electron-electron repulsion HOW MANY
th term:
1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
10
4Lecture
what is missing?
The Schrödinger Equation: Electron-Electron
Solve Schrödinger Equation HelΨ=EΨ
1 1 22
1Z A2Z B Z A Z B Z A
ZA 1
1
H
=
el Ai i
2M
i 2
A
i B2
A
RAB A B Ri ABA RAii
R r
A i jAi ij i j
rij
For benzene we have 12 nuclear
degrees of freedom (dof) and 42
electronic dof
For each set of nuclear dof, we solve HelΨ=EΨ to
calculate Ψ, the probability amplitudes for finding the 42
electrons at various locations
1st term: kinetic energy operator: 42 terms
2nd term: attraction of electrons to nuclei: 42*12= 504
3rd term: electron-electron repulsion 42*41/2= 861 terms
th term:
1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
11
4Lecture
what is missing?
The Schrödinger Equation: Nuclear-Nuclear
Solve Schrödinger Equation HelΨ=EΨ
1 1 22
1Z A2Z B Z A Z B Z A
ZA 1
1
H
=
2
i
el Ai
M
2
R
R
2
R
R
r
i B
i
A i jAi ij i j rij
i A
A
AB A B i AB A
Ai
Missing is the nuclear-nuclear repulsion
Enn = SA<B ZA*ZB/RAB
This does not depend on electron coordinates, but it
does affect the total energy
Eel = <Ψ|Hel|Ψ>/<Ψ|Ψ>=
Etotal = Eel + Enn
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
12
The supersonic review of QM
You should have already been exposed to all the
material on the next xx slides
This is just a review to remind you of the key points
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
13
Quantum Mechanics – First postulate
The essential element of QM is that all properties that can
be known about the system are contained in the
wavefunction, Φ(x,y,z,t) (for one electron), where the
probability of finding the electron at position x,y,z at time t
is given by
P(x,y,z,t) = | Φ(x,y,z,t) |2 = Φ(x,y,z,t)* Φ(x,y,z,t)
Note that ∫Φ(x,y,z,t)* Φ(x,y,z,t) dxdydz = 1
since the total probability of finding the electron
somewhere is 1.
I write this as < Φ|Φ>=1, where it is understood that the
integral is over whatever the spatial coordinates of Φ are
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
14
Quantum Mechanics – Second postulate
In QM the total energy can be written as
EQM = KEQM + PEQM
where for a system with a classical potential energy function,
V(x,y,z,t)
PEQM=∫Φ(x,y,z,t)*V(x,y,z,t)Φ(x,y,z,t)dxdydz ≡ < Φ| V|Φ>
Just like Classical mechanics except that V is weighted by P=|Φ|2
For the H atom
_
2/r) |Φ> = -e2/ R
PEQM=<
Φ|
(-e
_
where R is the average value of 1/r
KEQM = (Ћ2/2me) <(Φ·Φ>
where <(Φ·Φ> ≡ ∫ [(dΦ/dx)2 + (dΦ/dx)2 + (dΦ/dx)2] dxdydz
In QM the KE is proportional to the average square of the gradient
or slope of the wavefunction
Thus
KE wants smooth© wavefunctions,
no wiggles
Lecture 1Ch121a-Goddard-L01
copyright 2011 William A. Goddard
III, all rights reserved\
15
Summary 2nd Postulate QM
EQM = KEQM + PEQM
where for a system with a potential energy function, V(x,y,z,t)
PEQM= < Φ| V|Φ>=∫Φ(x,y,z,t)*V(x,y,z,t)Φ(x,y,z,t)dxdydz
Just like Classical mechanics except weight V with P=|Φ|2
KEQM = (Ћ2/2me) <(Φ·Φ>
where <(Φ·Φ> ≡ ∫ [(dΦ/dx)2 + (dΦ/dx)2 + (dΦ/dx)2] dxdydz
We have assumed a normalized wavefunction, <Φ|Φ> = 1
The stability of the H atom was explained by this KE (proportional
to the average square of the gradient of the wavefunction).
Also we used the preference of KE for smooth wavefunctions to
explain the bonding in H2+ and H2.
So far we have been able to use the above expressions to reason
qualitatively. However to actually solve for the wavefunctions
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
16
requires
the Schrodinger
Eqn., which we derive next.
3rd Postulate of QM, the variational principle
The ground state wavefunction is the system, Φ, has the lowest
possible energy out of all possible wavefunctions.
Consider that Φex is the exact wavefunction with energy
Eex = <Φ’|Ĥ|Φ’>/<Φ’|Φ’> and that
Φap = Φex + dΦ is some other approximate wavefunction.
Then Eap = <Φap|Ĥ|Φap>/<Φap|Φap> ≥ Eex
This means that for sufficiently small
dΦ, dE = 0 for all possible changes,
dΦ
We write dE/dΦ = 0 for all dΦ
E
Eex
Eap
This is called the variational principle.
For the ground state, d2E/dΦ ≥ 0 for all
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
possible
changes
17
4th postulate of QM
Consider the exact eigenstate of a system
HΦ = EΦ
and multiply the Schrödinger equation by some CONSTANT
phase factor (independent of position and time)
exp(ia) = eia
eia HΦ = H (eia Φ) = E (eia Φ)
Thus Φ and (eia Φ) lead to identical properties and we
consider them to describe exactly the same state.
4th Postulate: wavefunctions differing only by a constant
phase factor describe the same state
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
18
Electron spin, 5th postulate QM
Consider application of a magnetic field
Our Hamiltonian has no terms dependent on the magnetic field.
Hence no effect.
But experimentally there is a huge effect. Namely
The ground state of H atom splits into two states
b
B=0
Increasing B
a
This leads to the 5th postulate of QM
In addition to the 3 spatial coordinates x,y,z each electron has
internal or spin coordinates that lead to a magnetic dipole aligned
either with the external magnetic field or opposite.
We label these as a for spin up and b for spin down. Thus the
ground states of H atom are φ(xyz)a(spin) and φ(xyz)b(spin)
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
19
Permutational symmetry, summary
Our Hamiltonian for H2,
H(1,2) =h(1) + h(2) + 1/r12 + 1/R
Does not involve spin
This it is invariant under 3 kinds of permutations
Space only: r1 r2
Spin only: s1 s2
Space and spin simultaneously: (r1,s1) (r2,s2)
Since doing any of these interchanges twice leads to the identity,
we know from previous arguments that
Ψ(2,1) = Ψ(1,2) symmetry for transposing spin and space coord
Φ(2,1) = Φ(1,2) symmetry for transposing space coord
Χ(2,1) = Χ(1,2) symmetry for transposing spin coord
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
20
Permutational symmetries for H2 and He
H2
Have 4 degenerate g
ground states for H2
Have 4 degenerate u
excited states for H2
He
Have 4 degenerate
ground state for He
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
21
Permutational symmetries for H2 and He
H2
He
Lecture 1Ch121a-Goddard-L01
the only states
observed are
those for
which the
wavefunction
changes sign
upon
transposing all
coordinates of
electron 1 and
2
Leads to the
6th postulate of
© copyright 2011 William A. Goddard III, all rights reserved\
22
QM
The 6th postulate of QM: the Pauli Principle
For every eigenstate of an electronic system
H(1,2,…i…j…N)Ψ(1,2,…i…j…N) = EΨ(1,2,…i…j…N)
The electronic wavefunction Ψ(1,2,…i…j…N) changes
sign upon transposing the total (space and spin)
coordinates of any two electrons
Ψ(1,2,…j…i…N) = - Ψ(1,2,…i…j…N)
We can write this as
tij Ψ = - Ψ for all I and j
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
23
Consider H atom
We will consider one electron, but a nucleus with charge Z
r
The Hamiltonian has the form
h = - (Ћ2/2me)2 – Ze2/r
In atomic units
h = - ½ 2 – Z/r
+Ze
Thus we want to solve
Hφk = ekφk for the ground and excited states k
φnlm = Rnl(r) Zlm(θ,φ)
where Rnl(r) depends only on r and
Zlm(θ,φ) depends only on θ and φ
Assume φ10 = exp(-zr) E = ½ z2 – Z z
dE/dz
= z – Z = 0 z =© copyright
Z
Lecture 1Ch121a-Goddard-L01
2011 William A. Goddard III, all rights reserved\
24
The H atom ground state
the ground state of H atom is
φ1s = N0 exp(-Zr/a0) ~ exp(-Zr) where we will ignore normalization
1
x=0
Line plot along z, through the origin
Contour plot in the xz
plane, Maximum
Maximum amplitude at z = 0
amplitude at x,z = 0,0
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
25
Atomic units
We will use atomic units for which me = 1, e = 1, Ћ = 1
For H atom the average size of the 1s orbital is
a0 = Ћ2/ mee2 = 0.529 A =0.053 nm = 1 au is the unit of length
For H atom the energy of the 1s orbital []ionization potential (IP) of
H atom is
e1s = - ½ me e4/ Ћ2 = - ½ h0 = -13.61 eV = 313.75 kcal/mol
In atomic units the unit of energy is me e4/ Ћ2 = h0 = 1, denoted as
the Hartree
Note h0 = e2/a0 = 27.2116 eV = 627.51 kcal/mol = 2625.5 kJ/mol
Thus e1s = The kinetic energy of the 1s state is KE1s = ½ and
the potential energy is PE1s = -1 = 1/ where = 1 a0 is the
average radius
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
26
The excited states of H atom - 1
The ground and excited states of a system can all be written as
hφk = ekφk, where <φk |φj> = dkj (0 when j=k, 0 otherwise)
We say that they are orthogonal. Nodal Theorem ground state
has no nodes (never changes sign), like the 1s state for H atom
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
27
The excited states of H atom - 2
The ground and excited states of a system can all be written as
hφk = ekφk, where <φk |φj> = dkj (0 when j=k, 0 otherwise)
We say that they are orthogonal. Nodal Theorem ground state
has no nodes (never changes sign), like the 1s state for H atom
z
Use spherical polar coordinates, r, θ, φ
θ
where z = rcosθ, x = rsinθcosφ, y = rsinθsinφ
y
2 = d2/dx2 + d2/dy2 + d2/dy2 transforms like
x
r2 = x2 + y2 + z2 so that it is independent of θ, φ φ
Thus h(r,θ,φ) = - ½ 2 – Z/r is independent of θ
and φ
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
28
The excited states of H atom - 3
The ground and excited states of a system can all be written as
hφk = ekφk, where <φk |φj> = dkj (0 when j=k, 0 otherwise)
We say that they are orthogonal. Nodal Theorem ground state
has no nodes (never changes sign), like the 1s state for H atom
z
Use spherical polar coordinates, r, θ, φ
θ
where z=r cosθ, x=r sinθ cosφ, y=r sinθ sinφ
y
2 = d2/dx2 + d2/dy2 + d2/dy2 transforms like
x
r2 = x2 + y2 + z2 so that it is independent of θ, φ φ
Thus h(r,θ,φ) = - ½ 2 – Z/r is independent of θ
and φ
Consequently the eigenfunctions of h can be factored into Rnl(r)
depending only on r and Zlm(θ,φ) depending only on θ and φ
φnlm = Rnl(r) Zlm(θ,φ)
The reason for the numbers nlm will be apparent later
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
29
Excited radial functions
Consider excited states with Znl = 1; these are ns states with l=0
The lowest is R10 = 1s = exp(-Zr), the ground state.
All other radial functions must be orthogonal to 1s, and hence
must have one or more radial nodes.
0 nodal
1 spherical
2 spherical
planes
nodal plane
nodal planes
Zr = 7.1
Zr = 2
Zr = 1.9
The cross section is plotted along the z axis, but it would look
exactly the same along any other axis. Here
R20 = 2s = [Zr/2 – 1] exp(-Zr/2)
R30 1Ch121a-Goddard-L01
= 3s = [2(Zr)2/27 ©–copyright
2(Zr)/3
1] exp(-Zr/3)
Lecture
2011+William
A. Goddard III, all rights reserved\
30
Angularly excited states
Ground state 1s = φ100 = R10(r) Z00(θ,φ), where Z00 = 1 (constant)
Now consider excited states, φnlm = Rnl(r) Zlm(θ,φ), whose angular
functions, Zlm(θ,φ), are not constant, l ≠ 0.
Assume that the radial function is smooth, like R(r) = exp(-ar)
Then for the excited state to be orthogonal to the 1s state, we
must have
<Z00(θ,φ)|Zlm(θ,φ)> = 0
Thus Zlm must have nodal planes with equal positive and negative
regions.
The simplest cases are
rZ10 = z = r cosθ, which is zero in the xy plane
rZ11 = x = r sinθ cosφ, which is zero in the yz plane
rZ1,-1 = y = r sinθ sinφ, which is zero in the xz plane
These are denoted as angular momentum l=1 or p states
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
31
The p excited angular states of H atom
φnlm = Rnl(r) Zlm(θ,φ)
z
Now lets consider excited angular functions, Zlm.
They must have nodal planes to be orthogonal to Z00 +
The simplest would be Z10=z = r cosθ, which is
zero in the xy plane.
Exactly equivalent are
Z11=x = rsinθcosφ which is zero in the yz plane,
and
Z1-1=y = rsinθsinφ, which is zero in the xz plane
Also it is clear that these 3 angular functions
with one angular nodal plane are orthogonal to
each other. Thus <Z10|Z11> = <pz|px>=0 since
the integrand has nodes in both the xy and xz
planes, leading to a zero integral
pz
Lecture 1Ch121a-Goddard-L01
pz
x
z
px
-
+
x
z
-
+
+
-
© copyright 2011 William A. Goddard III, all rights reserved\
pxpz
x32
More p functions?
So far we have the s angular function Z00 = 1 with no angular
nodal planes
And three p angular functions: Z10 =pz, Z11 =px, Z1-1 =py, each
with one angular nodal plane
Can we form any more angular functions with one nodal plane
orthogonal to all 4 of the above functions?
z px’
For example we might rotate px by an angle a
a +
about the y axis to form px’. However multiplying,
say by pz, leads to the integrand pzpx’ which
clearly does not integrate to zero
x
z pzpx’
a +
-
Thus there are exactly three pi functions, Z1m,
. with m=0,+1,-1, all of which have the same KE.
Since the p functions have nodes, they lead to a
higher KE than the s function (assuming no
x radial©nodes)
+ 1Ch121a-Goddard-L01
Lecture
copyright 2011 William A. Goddard III, all rights reserved\
33
More angular functions?
So far we have the s angular function Z00 = 1 with no angular
nodal planes
And three p angular functions: Z10 =pz, Z11 =px, Z1-1 =py, each with
one angular nodal plane
Next in energy will be the d functions with two angular nodal
planes. We can easily construct three functions
dxy = xy =r2 (sinθ)2 cosφ sinφ
z
dyz = yz =r2 (sinθ)(cosθ) sinφ
dxz
+
2
dzx = zx =r (sinθ)(cosθ) cosφ
where dxz is shown here
+
-
x
Each of these is orthogonal to each other (and to the s and the
three p functions). <dxy|dyz> = ʃ (x z y2) = 0, <px|dxz> = ʃ (z x2) = 0,
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
34
More d angular functions?
In addition we can construct three other functions with two
nodal planes
z
dz2-x2
2
2
2
2
2
2
dx2-y2 = x – y = r (sinθ) [(cosφ) – (sinφ) ]
+
dy2-z2 = y2 – z2 = r2 [(sinθ)2(sinφ)2 – (cosθ)2]
dz2-x2 = z2 – x2 = r2 [(cosθ)2 -(sinθ)2(cosφ)2]
x
+
where dz2-x2 is shown here,
Each of these three is orthogonal to the previous three d
functions (and to the s and the three p functions)
This leads to six functions with 2 nodal planes
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
35
More d angular functions?
In addition we can construct three other functions with two
nodal planes
z
dz2-x2
2
2
2
2
2
2
dx2-y2 = x – y = r (sinθ) [(cosφ) – (sinφ) ]
+
dy2-z2 = y2 – z2 = r2 [(sinθ)2(sinφ)2 – (cosθ)2]
dz2-x2 = z2 – x2 = r2 [(cosθ)2 -(sinθ)2(cosφ)2]
x
+
where dz2-x2 is shown here,
Each of these three is orthogonal to the previous three d
functions (and to the s and the three p functions)
This leads to six functions with 2 nodal planes
However adding these 3 (x2 – y2) + (y2 – z2) + (z2 – x2) = 0
Which indicates that there are only two independent such
functions. We combine the 2nd two as
(z2 – x2) - (y2 – z2) = [2 z2 – x2 - y2 ] = [3 z2 – x2 - y2 –z2] =
= [3 1Ch121a-Goddard-L01
z2 – r2 ] which we ©denote
asWilliam
dz2 A. Goddard III, all rights reserved\
Lecture
copyright 2011
36
Summarizing the d angular functions
z
dz2
+
Z20 = dz2 = [3 – ]
m=0, ds
Z21 = dzx = zx =r2 (sinθ)(cosθ) cosφ
m = 1, dp
Z2-1 = dyz = yz =r2 (sinθ)(cosθ) sinφ
Z22 = dx2-y2 = x2 – y2 = r2 (sinθ)2 [(cosφ)2 – (sinφ)2]
Z22 = dxy = xy =r2 (sinθ)2 cosφ sinφ
We find it useful to keep track of how often the
wavefunction changes sign as the φ coordinate is
increased by 2p = 360º
When this number, m=0 we call it a s function
When m=1 we call it a p function
When m=2 we call it a d function
When m=3 we call it a f function
z2
r2
Lecture 1Ch121a-Goddard-L01
-
-
+
57º
x
m = 2, dd
© copyright 2011 William A. Goddard III, all rights reserved\
37
Summarizing the angular functions
So far we have
•one s angular function (no angular nodes) called ℓ=0
•three p angular functions (one angular node) called ℓ=1
•five d angular functions (two angular nodes) called ℓ=2
Continuing we can form
•seven f angular functions (three angular nodes) called ℓ=3
•nine g angular functions (four angular nodes) called ℓ=4
where ℓ is referred to as the angular momentum quantum number
And there are (2ℓ+1) m values for each ℓ
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
38
real (Zlm) and complex (Ylm) ang. momentum fnctns
Here the bar over
m negative
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
39
Size (a0)
name
total nodal planes
radial nodal planes
angular nodal planes
Combination of radial and angular nodal
planes
Combining radial and angular functions gives the
various excited states of the H atom. They are
named as shown where the n quantum number is
the total number of nodal planes plus 1
The nodal theorem does not specify how 2s and
1s 0 0 0 1.0
2p are related, but it turns out that the total
2s 1 1 0 4.0
energy depends only on n.
2p 1 0 1 4.0
3s 2 2 0 9.0 Enlm = - Z2/2n2
3p 2 1 1 9.0
The potential energy is given by
3d 2 0 2 9.0
4s 3 3 0 16.0 PE = - Z2/2n2 = -Z/ Rˉ , where Rˉ =n2/Z
4p 3 2 1 16.0
Thus Enlm = - Z/2 Rˉ
4d 3 1 2 16.0
all you
toGoddard
remember
Lecture
2011need
William A.
III, all rights reserved\
40
4f
3 1Ch121a-Goddard-L01
0 3 16.0 This ©iscopyright
Sizes hydrogen orbitals
Rˉ =a0 n2/Z
Where a0 = bohr = 0.529A=0.053 nm = 52.9 pm
Hydrogen orbitals 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f
Angstrom (0.1nm) 0.53, 2.12,
H
H C
H--H
H
H
0.74
1.7
4.77,
8.48
H
H
H
H
H
H
4.8
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
41
Hydrogen atom excited states
Energy
zero
-0.033 h0 = -0.9 eV
-0.056 h0 = -1.5 eV
-0.125 h0 = -3.4 eV
4s
4p
4d
3s
3p
3d
2s
2p
4f
Enlm = - Z/2 Rˉ = - Z2/2n2
-0.5 h0 = -13.6 eV
Lecture 1Ch121a-Goddard-L01
1s
© copyright 2011 William A. Goddard III, all rights reserved\
42
Plotting of orbitals:
line cross-section vs. contour
line plot
along z axis
Lecture 1Ch121a-Goddard-L01
contour plot
in yz plane
© copyright 2011 William A. Goddard III, all rights reserved\
43
Contour plots of 1s, 2s, 2p hydrogenic orbitals
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
44
Contour plots of 3s, 3p, 3d hydrogenic orbitals
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
45
Contour plots of 4s, 4p, 4d hydrogenic orbtitals
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
46
Contour plots of hydrogenic 4f orbitals
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
47
He+ atom
Next lets review the energy for He+.
Writing Φ1s = exp(-zr) we determine the optimum z for He+ as
follows
<1s|KE|1s> = + ½ z2 (goes as the square of 1/size)
<1s|PE|1s> = - Zz (linear in 1/size)
E(He+) = + ½ z2 - Zz
Applying the variational principle, the optimum z must satisfy
dE/dz = z - Z = 0 leading to z = Z,
KE = ½ Z2, PE = -Z2, E=-Z2/2 = -2 h0.
writing PE=-Z/R0, we see that the average radius is R0=1/z = 1/2
So that the He+ orbital is ½ the size of the H orbital
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
48
Estimate J1s,1s, the electron repulsion energy of 2
electrons in He+ 1s orbitals
e1
Now consider He atom: EHe = 2(½ z2) – 2Zz J1s,1s
How can we estimate J1s,1s
R0
e2
Assume that each electron moves on a sphere
With the average radius R0 = 1/z =1/2
And assume that e1 at along the z axis (θ=0)
Neglecting correlation in the electron motions, e2 will on the
average have θ=90º so that the average e1-e2 distance is
~sqrt(2)*R0
Thus J1s,1s ~ 1/[sqrt(2)*R0] = 0.707 z
A rigorous calculation gives
J1s,1s = (5/8) z = 0.625 z = (5/16) h0 = 8.5036 eV = 196.1 kcal/mol
Since e1s = -Z2/2 = -2 h0 = 54.43 eV = 1,254.8 kcal/mol the
Lecture 1Ch121a-Goddard-L01
© copyrightthe
2011 energy
William A. Goddard
all rights reserved\
electron
repulsion increases
(lessIII,attractive)
by 15.6%49
The optimum atomic orbital for He atom
He atom: EHe = 2(½ z2) – 2Zz (5/8)z
Applying the variational principle, the optimum z must satisfy
dE/dz = 0 leading to
2z - 2Z + (5/8) = 0
Thus z = (Z – 5/16) = 1.6875
KE = 2(½ z2) = z2
PE = - 2Zz (5/8)z = -2 z2
E= - z2 = -2.8477 h0
Ignoring e-e interactions the energy would have been E = -4 h0
The exact energy is E = -2.9037 h0 (from memory, TA please
check).
Thus this simple approximation of assuming that each electron is
in a 1s orbital and optimizing the size accounts for 98.1% of the
exact result.
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
50
Interpretation: The optimum atomic orbital for He atom
Assume He(1,2) = Φ1s(1)Φ1s(2) with Φ1s = exp(-zr)
We find that the optimum z = (Z – 5/16) = Zeff = 1.6875
With this value of z, the total energy is E= - z2 = -2.8477 h0
This wavefunction can be interpreted as two electrons moving
independently in the orbital Φ1s = exp(-zr) which has been
adjusted to account for the average shielding due to the other
electron in this orbital.
On the average this other electron is closer to the nucleus about
31.25% of the time so that the effective charge seen by each
electron is Zeff = 2 - 0.3125=1.6875
The total energy is just the sum of the individual energies,
E = -2 (Zeff2/2) = -2.8477 h0
Ionizing the 2nd electron, the 1st electron readjusts to z = Z = 2
with E(He+) = -Z2/2 = - 2 h0.
Thus the ionization potential (IP) is 0.8477 h0 = 23.1 eV (exact
value1Ch121a-Goddard-L01
= 24.6 eV)
Lecture
© copyright 2011 William A. Goddard III, all rights reserved\
51
Now lets add a 3rd electron to form Li
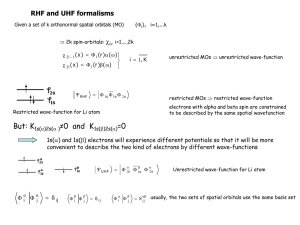
ΨLi(1,2,3) = A[(Φ1sa)(Φ1sb)(Φ1sg)]
Problem: with either g = a or g = b, we get ΨLi(1,2,3) = 0
This is an essential result of the Pauli principle
Thus the 3rd electron must go into an excited orbital
ΨLi(1,2,3) = A[(Φ1sa)(Φ1sb) )(Φ2sa)]
or
ΨLi(1,2,3) = A[(Φ1sa)(Φ1sb) )(Φ2pza)] (or 2px or 2py)
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
52
First consider Li+
First consider Li+ with ΨLi(1,2) = A[(Φ1sa)(Φ1sb)]
Here Φ1s = exp(-zr) with z = Z-0.3125 = 2.69 and
E = -z2 = -7.2226 h0.
For Li2+ we get E =-Z2/2=-4.5 h0
Thus the IP of Li+ is IP = 2.7226 h0 = 74.1 eV
The size of the 1s orbital for Li+ is
R0 = 1/z = 0.372 a0 = 0.2A
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
53
Consider adding the 3rd electron to the 2p orbital
ΨLi(1,2,3) = A[(Φ1sa)(Φ1sb) )(Φ2pza)] (or 2px or 2py)
Since the 2p orbital goes to zero at z=0, there is very
little shielding so that the 2p electron sees an effective
charge of
Zeff = 3 – 2 = 1, leading to
a size of R2p = n2/Zeff = 4 a0 = 2.12A
And an energy of e = -(Zeff)2/2n2 = -1/8 h0 = 3.40 eV
1s
0.2A
2p
2.12A
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
54
First consider Li+
First consider Li+ with ΨLi(1,2) = A[(Φ1sa)(Φ1sb)]
Here Φ1s = exp(-zr) with z = Z-0.3125 = 2.69 and
E = -z2 = -7.2226 h0.
For Li2+ we get E =-Z2/2=-4.5 h0
Thus the IP of Li+ is IP = 2.7226 h0 = 74.1 eV
The size of the 1s orbital for Li+ is
R0 = 1/z = 0.372 a0 = 0.2A
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
55
Consider adding the 3rd electron to the 2p orbital
ΨLi(1,2,3) = A[(Φ1sa)(Φ1sb) )(Φ2pza)] (or 2px or 2py)
Since the 2p orbital goes to zero at z=0, there is very
little shielding so that the 2p electron sees an effective
charge of
Zeff = 3 – 2 = 1, leading to
a size of R2p = n2/Zeff = 4 a0 = 2.12A
And an energy of e = -(Zeff)2/2n2 = -1/8 h0 = 3.40 eV
1s
0.2A
2p
2.12A
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
56
Consider adding the 3rd electron to the 2s orbital
ΨLi(1,2,3) = A[(Φ1sa)(Φ1sb) )(Φ2pza)] (or 2px or 2py)
The 2s orbital must be orthogonal to the 1s, which means that
it must have a spherical nodal surface at ~ 0.2A, the size of the
1s orbital. Thus the 2s has a nonzero amplitude at z=0 so that
it is not completely shielded by the 1s orbitals.
The result is Zeff2s = 3 – 1.72 = 1.28
This leads to a size of R2s = n2/Zeff = 3.1 a0 = 1.65A
And an energy of e = -(Zeff)2/2n2 = -0.205 h0 = 5.57 eV
1s
0.2A
2s
Lecture 1Ch121a-Goddard-L01
2.12A
R~0.2A
© copyright 2011 William A. Goddard III, all rights
reserved\
57
Li atom excited states
Energy
MO picture
State picture
zero
1st
excited
state
-0.125 h0 = -3.4 eV
-0.205 h0 = -5.6 eV
2p
2s
DE = 2.2 eV
17700 cm-1
564 nm
(1s)2(2p)
(1s)2(2s)
Ground
state
Exper
-2.723 h0 = 74.1 eV
671 nm
1s
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
DE = 1.9 eV58
Aufbau principle for atoms
Energy
Kr, 36
Zn, 30
Ar, 18
6
2
2
4s
10
4p
10
6
14
4d
4f
3d
3p
3s
Ne, 10
6
2p
Get generalized energy
spectrum for filling in the
electrons to explain the
periodic table.
2
2s
2
Full shells at 2, 10, 18, 30,
36 electrons
1s
© copyright 2011 William A. Goddard III, all rights reserved\
He, 2
Lecture 1Ch121a-Goddard-L01
59
Kr, 36
Zn, 30
Ar, 18
Ne, 10
He, 2
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
60
Many-electron configurations
General
aufbau
ordering
Lecture 1Ch121a-Goddard-L01
Particularly stable
© copyright 2011 William A. Goddard III, all rights reserved\
61
General trends along a row of the periodic table
As we fill a shell, say B(2s)2(2p)1 to Ne (2s)2(2p)6
we add one more proton to the nucleus and one more electron to
the valence shell
But the valence electrons only partially shield each other.
Thus Zeff increases, leading to a decrease in the radius ~ n2/Zeff
And an increase in the IP ~ (Zeff)2/2n2
Example Zeff2s=
1.28 Li, 1.92 Be, 2.28 B, 2.64 C, 3.00 N, 3.36 O, 4.00 F, 4.64 Ne
Thus (2s Li)/(2s Ne) ~ 4.64/1.28 = 3.6
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
62
General trends along a column of the periodic
table
As we go down a column
Li [He}(2s) to Na [Ne]3s to K [Ar]4s to Rb [Kr]5s to Cs[Xe]6s
We expect that the radius ~ n2/Zeff
And the IP ~ (Zeff)2/2n2
But the Zeff tends to increase, partially compensating for
the change in n so that the atomic sizes increase only
slowly as we go down the periodic table and
The IP decrease only slowly (in eV):
5.39 Li, 5.14 Na, 4.34 K, 4.18 Rb, 3.89 Cs
(13.6 H), 17.4 F, 13.0 Cl, 11.8 Br, 10.5 I, 9.5 At
24.5 He, 21.6 Ne, 15.8 Ar, 14.0 Kr,12.1 Xe, 10.7 Rn
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
63
Plot of rφ(r) for
the outer s
valence orbital
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
64
Plot of rφ(r) for
the outer s and
p valence
orbitals
Note for C row
2s and 2p have
similar size, but
for other rows
ns much
smaller than np
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
65
Plot of rφ(r) for the
outer s and p valence
orbitals
Note for C row 2s
and 2p have similar
size, but for other
rows ns much
smaller than np
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
66
Transition metals; consider [Ar] + 1 electron
[IP4s = (Zeff4s )2/2n2 = 4.34 eV Zeff4s = 2.26; 4s<4p<3d
K
IP4p = (Zeff4p )2/2n2 = 2.73 eV Zeff4p = 1.79;
IP3d = (Zeff3d )2/2n2 = 1.67 eV Zeff3d = 1.05;
IP4s = (Zeff4s )2/2n2 = 11.87 eV Zeff4s = 3.74; 4s<3d<4p
Ca+
IP3d = (Zeff3d )2/2n2 = 10.17 eV Zeff3d = 2.59;
IP4p = (Zeff4p )2/2n2 = 8.73 eV Zeff4p = 3.20;
IP3d = (Zeff3d )2/2n2 = 24.75 eV Zeff3d = 4.05; 3d<4s<4p
Sc++
IP4s = (Zeff4s )2/2n2 = 21.58 eV Zeff4s = 5.04;
IP4p = (Zeff4p )2/2n2 = 17.01 eV Zeff4p = 4.47;
As the net charge increases the differential shielding for 4s vs 3d
is less important than the difference in n quantum number 3 vs 4
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
67
Thus
charged system prefers
3d vs 4s
Transition metals; consider Sc0, Sc+, Sc2+
3d: IP3d = (Zeff3d )2/2n2 = 24.75 eV Zeff3d = 4.05;
Sc++
4s: IP4s = (Zeff4s )2/2n2 = 21.58 eV Zeff4s = 5.04;
4p: IP4p = (Zeff4p )2/2n2 = 17.01 eV Zeff4p = 4.47;
(3d)(4s): IP4s = (Zeff4s )2/2n2 = 12.89 eV Zeff4s = 3.89;
Sc+
(3d)2:
IP3d = (Zeff3d )2/2n2 = 12.28 eV Zeff3d = 2.85;
(3d)(4p): IP4p = (Zeff4p )2/2n2 = 9.66 eV Zeff4p = 3.37;
(3d)(4s)2: IP4s = (Zeff4s )2/2n2 = 6.56 eV Zeff4s = 2.78;
Sc
(4s)(3d)2: IP3d = (Zeff3d )2/2n2 = 5.12 eV Zeff3d = 1.84;
(3d)(4s)(4p): IP4p = (Zeff4p )2/2n2 = 4.59 eV Zeff4p = 2.32;
As increase net charge increases, the differential shielding for 4s
vs 3d is less important than the difference in n quantum number 3
vs 4. Thus M2+ transition metals always have all valence
electrons
in d orbitals © copyright 2011 William A. Goddard III, all rights reserved\
Lecture
1Ch121a-Goddard-L01
68
Implications on transition metals
The simple Aufbau principle puts 4s below 3d
But increasing the charge tends to prefers 3d vs 4s.
Thus Ground state of Sc 2+ , Ti 2+ …..Zn 2+ are all (3d)n
For all neutral elements K through Zn the 4s orbital is
easiest to ionize.
This is because of increase in relative stability of 3d for
higher ions
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
69
Transtion metal valence ns and (n-1)d orbitals
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
70
Review over, back to quantum mechanics
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
71
Excited states
The Schrödinger equation Ĥ Φk = EkΦk
Has an infinite number of solutions or eigenstates (German
for characteristic states), each corresponding to a possible
exact wavefunction for an excited state
For example H atom: 1s, 2s, 2px, 3dxy etc
Also the g and u states of H2+ and H2.
These states are orthogonal: <Φj|Φk> = djk= 1 if j=k
= 0 if j≠k
Note < Φj| Ĥ|Φk> = Ek < Φj|Φk> = Ek djk
We will denote the ground state as k=0
The set of all eigenstates of Ĥ is complete, thus any arbitrary
function Ө can be expanded as
Ө = S C Φ where <Φ
or Ө = Sk ΦIII,k all
<Φ
j| Ө>=C
k| Ө>
© copyright
2011j William A. Goddard
rights
reserved\
k k k
Lecture 1Ch121a-Goddard-L01
72
Quick fix to satisfy the Pauli Principle
Combine the product wavefunctions to form a symmetric
combination
Ψs(1,2)= ψe(1) ψm(2) + ψm(1) ψe(2)
And an antisymmetric combination
Ψa(1,2)= ψe(1) ψm(2) - ψm(1) ψe(2)
We see that
t12 Ψs(1,2) = Ψs(2,1) = Ψs(1,2) (boson symmetry)
t12 Ψa(1,2) = Ψa(2,1) = -Ψa(1,2) (Fermion symmetry)
Thus for electrons, the Pauli Principle only allows the
antisymmetric combination for two independent
electrons
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
73
Implications of the Pauli Principle
Consider two independent electrons,
1 on the earth described by ψe(1)
and 2 on the moon described by ψm(2)
Ψ(1,2)= ψe(1) ψm(2)
And test whether this satisfies the Pauli Principle
Ψ(2,1)= ψm(1) ψe(2) ≠ - ψe(1) ψm(2)
Thus the Pauli Principle does NOT allow
the simple product wavefunction for two
independent electrons
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
74
Consider some simple cases: identical spinorbitals
Ψ(1,2)= ψe(1) ψm(2) - ψm(1) ψe(2)
Identical spinorbitals: assume that ψm = ψe
Then Ψ(1,2)= ψe(1) ψe(2) - ψe(1) ψe(2) = 0
Thus two electrons cannot be in identical spinorbitals
Note that if ψm = eia ψe where a is a constant phase
factor, we still get zero
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
75
Consider some simple cases: orthogonality
Consider the wavefunction
Ψold(1,2)= ψe(1) ψm(2) - ψm(1) ψe(2)
where the spinorbitals ψm and ψe are orthogonal
hence <ψm|ψe> = 0
Define a new spinorbital θm = ψm + l ψe (ignore normalization)
That is NOT orthogonal to ψe. Then
Ψnew(1,2)= ψe(1) θm(2) - θm(1) ψe(2) =
=ψe(1) θm(2) + l ψe(1) ψe(2) - θm(1) ψe(2) - l ψe(1) ψe(2)
= ψe(1) ψm(2) - ψm(1) ψe(2) =Ψold(1,2)
Thus the Pauli Principle leads to orthogonality of
spinorbitals for different electrons, <ψi|ψj> = dij = 1 if i=j
Lecture 1Ch121a-Goddard-L01
=0 if i≠j
© copyright 2011 William A. Goddard III, all rights reserved\
76
Consider some simple cases: nonuniqueness
Starting with the wavefunction
ψm
Ψold(1,2)= ψe(1) ψm(2) - ψm(1) ψe(2)
Consider the new spinorbitals θm and θe where
θm = (cosa) ψm + (sina) ψe
Note that <θi|θj> = dij
a
Then Ψnew(1,2)= θe(1) θm(2) - θm(1) θe(2) =
+(cosa)2 ψ (1)ψ (2) +(cosa)(sina) ψ (1)ψ (2) θm
ψe
θe = (cosa) ψe - (sina) ψm
e
m
e
e
-(sina)(cosa) ψm(1) ψm(2) - (sina)2 ψm(1) ψe(2)
θe
a
-(cosa)2 ψm(1) ψe(2) +(cosa)(sina) ψm(1) ψm(2)
-(sina)(cosa) ψe(1) ψe(2) +(sina)2 ψe(1) ψm(2)
[(cosa)2+(sina)2] [ψe(1)ψm(2) - ψm(1) ψe(2)] =Ψold(1,2)
Thus
linear combinations
of the
Ψ(1,2)77
Lecture 1Ch121a-Goddard-L01
© copyright
2011spinorbitals
William A. Goddarddo
III, allnot
rightschange
reserved\
Determinants
The determinant of a matrix is defined as
The determinant is zero if any two columns (or rows) are identical
Adding some amount of any one column to any other column
leaves the determinant unchanged.
Thus each column can be made orthogonal to all other
columns.(and the same for rows)
The above properties are just those of the Pauli Principle
Thus
we will take determinants
ofWilliam
ourA.wavefunctions.
Lecture 1Ch121a-Goddard-L01
© copyright 2011
Goddard III, all rights reserved\
78
The antisymmetrized wavefunction
Now put the spinorbitals into the matrix and take the determinant
Where the antisymmetrizer
determinant operator.
can be thought of as the
Similarly starting with the 3!=6 product wavefunctions of the form
The only combination satisfying the Pauil Principle is
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
79
Example:
Interchanging electrons 1 and 3 leads to
From the properties of determinants we know that interchanging
any two columns (or rows), that is interchanging any two
spinorbitals, merely changes the sign of the wavefunction
Guaranteeing that the Pauli Principle is always satisfied
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
80
Energy for 2-electron product wavefunction
Consider the product wavefunction
Ψ(1,2) = ψa(1) ψb(2)
And the Hamiltonian for H2 molecule
H(1,2) = h(1) + h(2) +1/r12 + 1/R
In the details slides next, we derive
E = < Ψ(1,2)| H(1,2)|Ψ(1,2)>/ <Ψ(1,2)|Ψ(1,2)>
E = haa + hbb + Jab + 1/R
where haa =<a|h|a>, hbb =<b|h|b> are just the 1 electron energies
Jab ≡ <ψa(1)ψb(2) |1/r12 |ψa(1)ψb(2)>=ʃ [ψa(1)]2 [ψb(1)]2/r12
represents the total Coulomb interaction between the electron
density ra(1)=| ψa(1)|2 and rb(2)=| ψb(2)|2
Since the integrand ra(1) rb(2)/r12 is positive for all positions of 1
and 2, the integral is positive, Jab > 0
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
81
Details in deriving energy: normalization
First, the normalization term is
<Ψ(1,2)|Ψ(1,2)>=<ψa(1)|ψa(1)><ψb(2) ψb(2)>
Which from now on we will write as
<Ψ|Ψ> = <ψa|ψa><ψb|ψb> = 1 since the ψi are normalized
Here our convention is that a two-electron function such as
<Ψ(1,2)|Ψ(1,2)> is always over both electrons so we need not put
in the (1,2) while one-electron functions such as <ψa(1)|ψa(1)> or
<ψb(2) ψb(2)> are assumed to be over just one electron and we
ignore the labels 1 or 2
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
82
Details of deriving energy: one electron termss
Using H(1,2) = h(1) + h(2) +1/r12 + 1/R
We partition the energy E = <Ψ| H|Ψ> as
E = <Ψ|h(1)|Ψ> + <Ψ|h(2)|Ψ> + <Ψ|1/R|Ψ> + <Ψ|1/r12|Ψ>
Here <Ψ|1/R|Ψ> = <Ψ|Ψ>/R = 1/R since R is a constant
<Ψ|h(1)|Ψ> = <ψa(1)ψb(2) |h(1)|ψa(1)ψb(2)> =
= <ψa(1)|h(1)|ψa(1)><ψb(2)|ψb(2)> = <a|h|a><b|b> =
≡ haa
Where haa≡ <a|h|a> ≡ <ψa|h|ψa>
Similarly <Ψ|h(2)|Ψ> = <ψa(1)ψb(2) |h(2)|ψa(1)ψb(2)> =
= <ψa(1)|ψa(1)><ψb(2)|h(2)|ψb(2)> = <a|a><b|h|b> =
≡ hbb
The remaining term we denote as
Jab ≡ <ψa(1)ψb(2) |1/r12 |ψa(1)ψb(2)> so that the total energy is
Lecture
© copyright 2011 William A. Goddard III, all rights reserved\
E
= h1Ch121a-Goddard-L01
aa + hbb + Jab + 1/R
83
The energy for an antisymmetrized product, A ψaψb
The total energy is that of the product plus the exchange term
which is negative with 4 parts
Eex=-< ψaψb|h(1)|ψb ψa >-< ψaψb|h(2)|ψb ψa >-< ψaψb|1/R|ψb ψa >
- < ψaψb|1/r12|ψb ψa >
The first 3 terms lead to < ψa|h(1)|ψb><ψbψa >+
<ψa|ψb><ψb|h(2)|ψa >+ <ψa|ψb><ψb|ψa>/R
But <ψb|ψa>=0
Thus all are zero
Thus the only nonzero term is the 4th term:
-Kab=- < ψaψb|1/r12|ψb ψa > which is called the exchange energy
(or the 2-electron exchange) since it arises from the exchange
term due to the antisymmetrizer.
Summarizing, the energy of the Aψaψb wavefunction for H2 is
E = haa + hbb + (Jab –Kab) + 1/R
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
84
The energy of the antisymmetrized wavefunction
The total electron-electron repulsion part of the energy for any
wavefunction Ψ(1,2) must be positive
Eee =∫ (d3r1)((d3r2)|Ψ(1,2)|2/r12 > 0
This follows since the integrand is positive for all positions of r1
and r2 then
We derived that the energy of the A ψa ψb wavefunction is
E = haa + hbb + (Jab –Kab) + 1/R
Where the Eee = (Jab –Kab) > 0
Since we have already established that Jab > 0 we can conclude
that
Jab > Kab > 0
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
85
Separate the spinorbital into orbital and spin parts
Since the Hamiltonian does not contain spin the spinorbitals can
be factored into spatial and spin terms.
For 2 electrons there are two possibilities:
Both electrons have the same spin
ψa(1)ψb(2)=[Φa(1)a(1)][Φb(2)a(2)]= [Φa(1)Φb(2)][a(1)a(2)]
So that the antisymmetrized wavefunction is
Aψa(1)ψb(2)= A[Φa(1)Φb(2)][a(1)a(2)]=
=[Φa(1)Φb(2)- Φb(1)Φa(2)][a(1)a(2)]
Also, similar results for both spins down
Aψa(1)ψb(2)= A[Φa(1)Φb(2)][b(1)b(2)]=
=[Φa(1)Φb(2)- Φb(1)Φa(2)][b(1)b(2)]
Since <ψa|ψb>= 0 = < Φa| Φb><a|a> = < Φa| Φb>
We see that the spatial orbitals for same spin must be orthogonal
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
86
Energy for 2 electrons with same spin
The total energy becomes
E = haa + hbb + (Jab –Kab) + 1/R
where haa ≡ <Φa|h|Φa> and hbb ≡ <Φb|h|Φb>
where Jab= <Φa(1)Φb(2) |1/r12 |Φa(1)Φb(2)>
We derived the exchange term for spin orbitals with same spin as
follows
Kab ≡ <ψa(1)ψb(2) |1/r12 |ψb(1)ψa(2)>
`````= <Φa(1)Φb(2) |1/r12 |Φb(1)Φa(2)><a(1)|a(1)><a(2)|a(2)>
≡ Kab
where Kab ≡ <Φa(1)Φb(2) |1/r12 |Φb(1)Φa(2)>
Involves only spatial coordinates.
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
87
Energy for 2 electrons with opposite spin
Now consider the exchange term for spin orbitals with opposite
spin
Kab ≡ <ψa(1)ψb(2) |1/r12 |ψb(1)ψa(2)>
`````= <Φa(1)Φb(2) |1/r12 |Φb(1)Φa(2)><a(1)|b(1)><b(2)|a(2)>
=0
Since <a(1)|b(1)> = 0.
Thus the total energy is
Eab = haa + hbb + Jab + 1/R
With no exchange term unless the spins are the same
Since <ψa|ψb>= 0 = < Φa| Φb><a|b>
There is no orthogonality condition of the spatial orbitals for
opposite spin electrons
In general we can have <Φa|Φb> =S, where the overlap S ≠ 0
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
88
Summarizing: Energy for 2 electrons
When the spinorbitals have the same spin,
Aψa(1)ψb(2)= A[Φa(1)Φb(2)][a(1)a(2)]
The total energy is
Eaa = haa + hbb + (Jab –Kab) + 1/R
But when the spinorbitals have the opposite spin,
Aψa(1)ψb(2)= A[Φa(1)Φb(2)][a(1)b(2)]=
The total energy is
Eab = haa + hbb + Jab + 1/R
With no exchange term
Thus exchange energies arise only for the case in
which both electrons have the same spin
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
89
Consider further the case for spinorbtials with opposite spin
The wavefunction
[Φa(1)Φb(2)-Φb(1)Φa(2)][a(1)b(2)+b(1)a(2)]
Leads directly to
3E
ab = haa + hbb + (Jab –Kab) + 1/R
Exactly the same as for
[Φa(1)Φb(2)-Φb(1)Φa(2)][a(1)a(2)]
[Φa(1)Φb(2)-Φb(1)Φa(2)][b(1)b(2)]
These three states are collectively referred to as the triplet state
and denoted as having spin S=1
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
90
Consider further the case for spinorbtials with opposite spin
The other combination leads to one state, referred to as the
singlet state and denoted as having spin S=0
[Φa(1)Φb(2)+Φb(1)Φa(2)][a(1)b(2)-b(1)a(2)]
For the ground state of a 2-electron system, Φa=Φb so we get
[Φa(1)Φa(2)][a(1)b(2)-b(1)a(2)] = A[Φa(1)a(1)] [Φa(2)b(2)]
Leading directly to
1Eaa = 2haa + Jaa + 1/R
This state is referred to as the closed shell single state and
denoted as having spin S=0
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
91
Re-examine He atom with spin and the Pauli Principle
Ψ(1,2) = A[(φ1s a) (φ1s b)]
E= 2 <1s|h|1s> + J1s,1s
Which is exactly what we assumed above when we
ignore spin and the Pauli Principle
So for 2 electrons nothing changes
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
92
Energy for 2-electron product wavefunction
Consider the product wavefunction
Ψ(1,2) = ψa(1) ψb(2)
And the Hamiltonian for H2 molecule
H(1,2) = h(1) + h(2) +1/r12 + 1/R
In the details slides next, we derive
E = < Ψ(1,2)| H(1,2)|Ψ(1,2)>/ <Ψ(1,2)|Ψ(1,2)>
E = haa + hbb + Jab + 1/R
where haa =<a|h|a>, hbb =<b|h|b> are just the 1 electron energies
Jab ≡ <ψa(1)ψb(2) |1/r12 |ψa(1)ψb(2)>=ʃ d3r1[ψa(1)]2 ʃd3r2[ψb(2)]2/r12 =
= ʃ [ψa(1)]2 Jb (1) = <ψa(1)| Jb (1)|ψa(1)>
Where Jb (1) = ʃ [ψb(2)]2/r12 is the Coulomb potential at 1 due to
the density distribution [ψb(2)]2
1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\ 2
93
JLecture
ab is the Coulomb repulsion between densities ra=[ψa(1)] and rb
The energy for an antisymmetrized product,
A ψ aψ b
The total energy is that of the product wavefunction plus the new
terms arising from exchange term which is negative with 4 parts
Eex=-< ψaψb|h(1)|ψb ψa >-< ψaψb|h(2)|ψb ψa >-< ψaψb|1/R|ψb ψa >
- < ψaψb|1/r12|ψb ψa >
The first 3 terms lead to < ψa|h(1)|ψb><ψbψa >+
<ψa|ψb><ψb|h(2)|ψa >+ <ψa|ψb><ψb|ψa>/R
But <ψb|ψa>=0
Thus all are zero
Thus the only nonzero term is the 4th term:
-Kab=- < ψaψb|1/r12|ψb ψa > which is called the exchange energy
(or the 2-electron exchange) since it arises from the exchange
term due to the antisymmetrizer.
Summarizing, the energy of the Aψaψb wavefunction for H2 is
E = haa + hbb + (Jab –Kab) + 1/R
Lecture 1Ch121a-Goddard-L01
One© new
term
fromA. the
antisymmetrizer
copyright
2011 William
Goddard
III, all rights reserved\
94
Summary electron-electron energies
Jab ≡ <ψa(1)ψb(2) |1/r12 |ψa(1)ψb(2)>=<ψa(1)| Jb (1)|ψa(1)>
is the total Coulomb interaction between the electron density
ra(1)=| ψa(1)|2 and rb(2)=| ψb(2)|2
Since the integrand ra(1) rb(2)/r12 is positive for all positions of 1
and 2, the integral is positive, Jab > 0
Here Jb (1) = ʃ [ψb(2)]2/r12 is the potential at 1 due to the density
distribution [ψb(2)]2
Kab=< ψaψb|1/r12|ψb ψa >= ʃ d3r1[ψa(1)ψb(1)] ] ʃd3r2[ψb(2) ψa(2)]]2/r12
= <ψa(1)| Kb (1)|ψa(1)>
Where Kb (1) ψa(1)] ] = ψb(1) ʃ [ψb(2)ψa(2)]2/r12 is an integral
operator that puts Kab into a form that looks like Jab. The
difference is that Jb (1) is a function but Kb (1) is an operator
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
95
Alternative form for electron-electron energies
It is commen to rewrite Jab as
Jab ≡ [ψa(1) ψa(1)|ψb(2)ψb(2)] where all the electron 1 stuff is on
the left and all the electron 2 stuff is on the right. Note that the
1/r12 is now understood
Similarly Kab= [ψa(1)ψb(1)|ψb(2)ψa(2)]
Here the numbers 1 and 2 are superflous so we write
Jab ≡ [ψaψa|ψbψb] = [aa|bb] since only the orbital names are
significant
Siimilarly
Kab ≡ [ψaψb|ψbψa] = [ab|ba]
Thus the total 2 electron energy is
Jab - Kab = [aa|bb] - [ab|ba]
But if a and b have opposite spin then the exchange term is zero
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
96
Consider the case of 4 e in 2 orbitals, a,b
Ψ(1,2,3,4) = A[(aa)(ab)(ba)(bb)]
E = 2 haa + 2 hbb + Enn + [2Jaa-Kaa] +2[2Jab-Kab] + [2Jbb-Kbb]
= 2 haa + 2 hbb + Enn + 2(aa|aa)-(aa|aa)+4(aa|bb)-2(ab|ba)
+2(bb|bb)-(bb|bb)
Where we see that the self-Coulomb and self-exchange can
cancel.
Now change φ1 to φ1 + dφ1 the change in the energy is
dE = 4<dφ1|h|φ1> + 4 <dφ1|2J1-K1|φ1> + 4 <dφ1|2J2-K2|φ1>
= 4 <dφ1|HHF|φ1>
Where HHF = h + Σj=1,2 [2Jj-Kj] is called the HF Hamiltonian
In the above expression we assume that φ1 was normalized,
<φ1|h|φ1> = 1.
Imposing this constraint (a Lagrange multiplier) leads to
<dφ1|HHF – l1|φ1> = 0 and <dφ2|HHF – l2|φ2> = 0
Thus the optimum orbitals satisfy HHFφk = lk φk the HF equations
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
97
Starting point for First Principles QM
Classical Mechanics
Energy = Kinetic energy + Potential energy
Kinetic energy =
+
A
1
1 2
Z Z
Z
1
p
2A +
p
i A B A
2M A
i 2
A B RAB
i
A R Ai
i j rij
electrons
atoms
1
1 2
Z AZ B
ZA
1
2
A 2M energy
i 2 =i
A
Potential
R
R
A B
i
A
i j rij
A
AB
Ai
Nucleus-Nucleus
repulsion
Nucleus-Electron
attraction
Electron-Electron
repulsion
Can optimize electron coordinates and momenta separately,
thus lowest energy: all p=0 KE =0
All electrons on nuclei: PE = - infinity
Makes for dull world
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
98
Starting point for First Principles
Ab Initio, quantum mechanics
The wavefunction Ψ(r1,r2,…,rN) contains all
information of system determine KE and PE
Energy = < Ψ|KE operator|Ψ> + < Ψ|PE operator|Ψ>
Kinetic energy op =
A
1
1
Z Z
Z
1
2A i2 A B A
2M A
i 2
A B RAB
i
A RAi
i j rij
atoms
electrons
1
1 2
Z AZ B
ZA
1
2
Potential
A 2M energy
i 2= i
A
R
R
A B
i
A
i j rij
A
AB
Ai
Optimize Ψ, get HelΨ=EΨ
11 22 1Z A2Z B Z A Z B Z A Z A 1
1
i
A H
i
2A =A
2i Mel
Ai B2 RAB A B iRABA R Ai
i
A i RjAirij i j rij
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
99
Schrodinger Equation
HelΨ=EΨ
1 1 22
1Z A2Z B Z A Z B Z A
ZA 1
1
2A
H
Ai
i
=
el
2
R
R
r
2
M
2
R
R
A
i B
i
A i jAi ij i j rij
i A
A
AB A B i AB A
Ai
Solving SE gives exact properties of molecules, solids,
enzymes, etc
History
H atom, Schrodinger 1925-26
H2 Simple (Valence bond) 1927, accurate 1937
C2H6 simple 1963, accurate 1980’s
2008: can get accurate wavefunctions for ~100-200
atoms
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
100
Closed shell Hartree Fock (HF)
For benzene with 42 electrons, the ground state HF wavefunction
has 21 doubly occupied orbitals, φ1,.. φi,.. φ21
And we want to determine the optimum shape and energy for
these orbitals
First consider the componets of the total energy
Σ i=1,21< φi|h|φi> from the 21 up spin orbitals
Σ i=1,21< φi|h|φi> from the 21 down spin orbitals
Σ I<j=1,21 [Jij – Kij] interactions between the 21 up spin orbitals
Σ I<j=1,21 [Jij – Kij] interactions between the 21 down spin orbitals
Σ I≠j=1,21 [Jij] interactions of the 21 up spin orbitals with the 21
down spin orbitals
Enn = Σ A<B=1,12 ZAZB/RAB nuclear-nuclear repulsion
Combining these terms leads to
E = Σ i=1,21 2< φi|h|φi> + Enn + Σ I≠j=1,21 2[2Jij-Kij] + Σ I=1,21 [2Jii]
But Jii = Kii so we can rewrite this as
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
101
The energy expression for closed shell HF
E = Σ i=1,21 2< φi|h|φi> + Enn + Σ I<j=1,21 2[2Jij-Kij] + Σ I=1,21 [Jii]
This says for any two different orbitals we get 4 coulomb
interactions and 2 exchange interactions, but the two electrons in
the same orbital only lead to a single Coulomb term
Since Jii = Kii (self coulomb = self exchange) we can write
E = Σ i=1,21 2< φi|h|φi> + Enn + Σ I≠j=1,21 [2Jij-Kij] + Σ I=1,21 [2Jii-Kii]
and hence
E = Σ i=1,21 2< φi|h|φi> + Enn + Σ I,j=1,21 [2Jij-Kij]
which is the final expression for Closed Shell HF
Now we need to apply the variational principle to find the
equations determining the optimum orbitals, the HF orbitals
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
102
Consider the case of 4 electrons in 2 orbitals
E = 2<φ1|h|φ1> + 2< φ2|h|φ2> + Enn
+ [2J11-K11] +2[2J12-K12] + [2J22-K22]
Here we can write Jij = (ii|jj) where the first two indices go with
electron 1 and the other two with electron 2
Also we write Jij = (ii|jj) = <i|Jj|i>, where Jj is the coulomb potetial
seen by electron 1 due to the electron in orbital j.
Thus if we change φ1 to φ1 + dφ1 the change in the energy is
dE = 4<dφ1|h|φ1> + 4 <dφ1|2J1-K1|φ1> + 4 <dφ1|2J2-K2|φ1>
= 4 <dφ1|HHF|φ1>
Where HHF = h + Σj=1,2 [2Jj-Kj] is called the HF Hamiltonian
In the above expression we assume that φ1 was normalized,
<φ1|h|φ1> = 1.
Imposing this constraint (a Lagrange multiplier) leads to
<dφ1|HHF – l1|φ1> = 0 and <dφ2|HHF – l2|φ2> = 0
Thus the optimum orbitals satisfy HHFφk = lk φk the HF equations
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
103
The general case of 2M electrons
For the general case the HF closed shell equations are
HHFφk = lk φk where we solve for k=1,M occupied orbitals
HHF = h + Σj=1,M [2Jj-Kj]
This is the same as the Hamiltonian for a one electron system
moving in the average electrostatic and exchange potential, 2Jj-Kj
due to the other N-1 = 2M-1 electrons
Problem: sum over 2Jj leads to 2M Coulomb terms, not 2M-1
This is because we added the self Coulomb and exchange terms
But (2Jk-Kk) φk = (Jk) φk so that these self terms cancel.
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
104
Analyze HF equations
The optimum orbitals for the 4 electron closed shell wavefunction
Ψ(1,2,3,4) = A[(aa)(ab)(ba)(bb)]
Are eigenstate of the HF equations
HHFφk = lk φk for k=1,2
where HHF = h + Σj=1,2 [2Jj-Kj]
This looks like a one-electron Hamiltonian but it involves the
average Coulomb potential of 2 electrons in φa plus 2 electrons in
φb plus exchange interactions with one electron in φa plus one
electron in φb
It seems wrong that there should be 4 coulomb interactions
whereas each electron sees only 3 other electrons and that there
are two exchange interactions whereas each electron sees only
one other with the same spin.
This arises because we added and subtracted a self term in the
total energy
Since
(Jk-Kk)φk = 0 there
spurious terms cancel.
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
105
Main practical applications of QM
Determine the Optimum geometric structure and
energies of molecules and solids
Determine geometric structure and energies of
reaction intermediates and transition states for
various reaction steps
Determine properties of the optimized
geometries: bond lengths, energies,
frequencies, electronic spectra, charges
Determine reaction mechanism: detailed
sequence of steps from reactants to products
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
106
The Matrix HF equations
The HF equations are actually quite complicated because Kj
is an integral operator, Kj φk(1) = φj(1) ʃ d3r2 [φj(2) φk(2)/r12]
The practical solution involves expanding the orbitals in terms
of a basis set consisting of atomic-like orbitals,
φk(1) = Σm Cm Xm, where the basis functions, {Xm, m=1, MBF}
are chosen as atomic like functions on the various centers
As a result the HF equations HHFφk = lk φk
Reduce to a set of Matrix equations
ΣjmHjmCmk = ΣjmSjmCmkEk
This is still complicated since the Hjm operator includes
Exchange terms
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
107
Minimal Basis set – STO-3G
For benzene the smallest possible basis set is to use a 1s-like
single exponential function, exp(-zr) called a Slater function,
centered on each the 6 H atoms and
C1s, C2s, C2pz, C2py, C2pz functions on each of the 6 C atoms
This leads to 42 basis functions to describe the 21 occupied MOs
and is refered to as a minimal basis set.
In practice the use of exponetial functions, such as exp(-zr),
leads to huge computational costs for multicenter molecules and
we replace these by an expansion in terms of Gaussian basis
functions, such as exp(-ar2).
The most popular MBS is the STO-3G set of Pople in which 3
gaussian functions are combined to describe each Slater function
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
108
Double zeta + polarization Basis sets – 6-31G**
To allow the atomic orbitals to contract as atoms are brought
together to form bonds, we introduce 2 basis functions of the
same character as each of the atomic orbitals:
Thus 2 each of 1s, 2s, 2px, 2py, and 2pz for C
This is referred to as double zeta. If properly chosen this leads to
a good description of the contraction as bonds form.
Often only a single function is used for the C1s, called split
valence
In addition it is necessary to provide one level higher angular
momentum atomic orbitals to describe the polarization involved in
bonding
Thus add a set of 2p basis functions to each H and a set of 3d
functions to each C.
The most popular such basis is referred to as 6-31G**
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
109
6-31G** and 6-311G**
6-31G** means that the 1s is described with 6 Gaussians,
the two valence basis functions use 3 gaussians for the
inner one and 1 Gaussian for the outer function
The first * use of a single d set on each heavy atom
(C,O etc)
The second * use of a single set of p functions on each
H
The 6-311G** is similar but allows 3 valence-like functions
on each atom.
There are addition basis sets including diffuse functions (+)
and additional polarization function (2d, f) (3d,2f,g), but
these will not be relvent to EES810
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
110
Effective Core Potentials (ECP, psuedopotentials)
For very heavy atoms, say starting with Sc, it is computationally
convenient and accurate to replace the inner core electrons
with effective core potentials
For example one might describe:
• Si with just the 4 valence orbitals, replacing the Ne core with
an ECP or
• Ge with just 4 electrons, replacing the Ni core
• Alternatively, Ge might be described with 14 electrons with the
ECP replacing the Ar core. This leads to increased accuracy
because the
• For transition metal atoms, Fe might be described with 8
electrons replacing the Ar core with the ECP.
• But much more accurate is to use the small Ne core, explicitly
treating the (3s)2(3p)6 along with the 3d and 4s electrons
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
111
Software packages
Jaguar: Good for organometallics
QChem: very fast for organics
Gaussian: many analysis tools
GAMESS
HyperChem
ADF
Spartan/Titan
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
112
Results for Benzene
The energy of the C1s orbital is ~ - Zeff2/2
where Zeff = 6 – 0.3125 = 5.6875
Thus e1s ~ -16.1738 h0 = - 440.12 eV.
This leads to 6 orbitals all with very similar energies.
This lowest has the + combination of all 6 1s orbitals,
while the highest alternates with 3 nodal planes.
There are 6 CH bonds and 6 CC bonds that are
symmetric with respect to the benzene plane, leading to
12 sigma MOs
The highest MOs involve the p electrons. Here there are
6 electrons and 6 pp atomic orbitals leading to 3 doubly
occupied and 3 empty orbitals with the pattern
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
113
Pi orbitals of benzene
Top view
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
114
The HF orbitals of
N2
With 14 electrons we
get M=7 doubly
occupied HF orbitals
We can visualize this
as a triple NN bond
plus valence lone
pairs
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
115
The energy diagram for N2
TAs put energies of 7
occupied orbitals plus
lowest 2 unoccupied
orbitals, use correct
symmetry notation
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
116
The HF orbitals of H2O
TAs put energies of 5
occupied orbitals plus
lowest 2 unoccupied
orbitals, use correct
symmetry notation
Show orbitals
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
117
The HF orbitals of ethylene
TAs put energies of 8
occupied orbitals plus
lowest 2 unoccupied
orbitals, use correct
symmetry notation
Show orbitals
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
118
The HF orbitals of benzene
TAs put energies of
21 occupied orbitals
plus lowest 4
unoccupied orbitals,
use correct symmetry
notation
Show orbitals
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
119
HF wavefunctions
Good distances, geometries, vibrational levels
But
breaking bonds is described extremely poorly
energies of virtual orbitals not good description of
excitation energies
cost scales as 4th power of the size of the
system.
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
120
Configuration interaction
Consider a set of N-electron wavefunctions: {i;
i=1,2, ..M} where < i|j> = dij {=1 if i=j and 0 if i ≠j)
Write approx = S (i=1 to M) Ci i
Then E = < approx|H|approx>/< approx|approx>
E= < Si Ci i |H| Si Cj j >/ < Si Ci i | Si Cj j >
How choose optimum Ci?
Require dE=0 for all dCi get
Sj <i |H| Cj j > - Ei< i | Cj j > = 0 ,which we
write as
HCi = SCiEi in matrix notation, ie ΣjkHjkCki = ΣjkSjkCkiEi
where Hjk = <j|H|k > and Sjk = < j|k > and Ci is a
column vector for the ith eigenstate
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
121
Configuration interaction upper bound theorm
Consider the M solutions of the CI equations
HCi = SCiEi ordered as i=1 lowest to i=M highest
Then the exact ground state energy of the system
Satisfies Eexact ≤ E1
Also the exact first excited state of the system
satisfies
E1st excited ≤ E2
etc
This is called the Hylleraas-Unheim-McDonald
Theorem
Lecture 1Ch121a-Goddard-L01
© copyright 2011 William A. Goddard III, all rights reserved\
122