Electron Configuration
advertisement

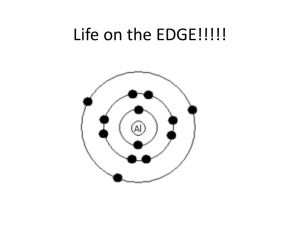
·http://www.youtube.com/watch?v=JNPFR22MPA ·http://www.chemguide.co.uk/atoms/propertie s/atomorbs.html ·http://www.chemguide.co.uk/atoms/propertie s/orbitsorbitals.html#top ·http://www.chemguide.co.uk/atoms/propertie s/elstructs.html#top 1 What Does an Atom Look Like? Draw what you think N looks like in the box below... What is wrong with this diagram? What do the circles really represent? 3 Why have we been taught circles...and that electrons travel in perfect orbits? ·Circles are not actually wrong, they are just misrepresented when they are taught by some people. ·The truth is that circles are meant to represent energy levels of the electrons around the nucleus, not necessarily the paths the "follow" around the nucleus. ·As electrons get further and further away from the nucleus, they have more and more energy, because they are less "drawn in" by the nucleus. Here is what electron clouds in an Atom really look like... http://www.youtube.com/watch?v=sMt5Dcex 0kg Electrons travel in Subshells, known as Orbitals. These are not ORBITS, but they are ORBITALS. The electron has a 95% probability that it is within its designated orbital Heisenberg Uncertainty Principle ·Says, loosely, that you can't know with certainty both where an electron is and where it's going next. ·What it actually says is that it is impossible to define with absolute precision, at the same time, both the position and the momentum of an electron.) 4 Types of Subshells P orbitals can hold 6 Electrons S orbitals can hold 2 Electrons S orbitals can hold 2 Electrons S orbitals can hold 2 Electrons 6 Electrons orbitals are represented by 4 shapes (S,P,D,F) p s group # = # valence (outside) 8A e3A 4A 5A 6A 7A 1A 1 2A d 2 Row 3 = 4 # shells 5 3B 4B 5B 6B 7B 8B 8B 8B 1B 2B s p d 6 7 6 f 7 f Notice something different with d? We Can represent the # of electrons in their shells in what is called- Electron 1s1 Configuration row # = shell # possibilities are 1-7 7 rows subshell- group # = # valence epossibilities are: s: 1 or 2 p: 1-6 s, p, d, or d: 1-10 f f: 1-14 4 Total e- should subshells equal Atomic #, Why? Lets try and figure out what shells are in Cl? Subshells d and f are “special”- They are each staggered by one row. So for row 4, they are actually 3d, not 4d! group # = # valence e- 1A 1 2A period # = # eshells 2 3 3B 4B 5B 6B 7B 8B 8B 8B 1B 2B 4 3d 4d 5d 6d 5 6 7 d 6 4f 7 5f f 8A 3A 4A 5A 6A 7A Try and write them out in order, you can stop with 6s. Order of Electron Subshell Filling: It does not go “in order”, you read the periodic table from left to right. 1s 2s2 2p6 3s2 3p6 4s2 3d 4p6 5s2 4d 5p6 6s2 4f 5d The Electrons in an atom can be show with letters and numbers (Electron Configuration), or also with boxes (Ground State Configuration). They show the same information, just a different representation. ·Electrons are represented here in boxes with up and down arrows. ·Electrons will try and "space" out in the available boxes, so you fill them one at a time. ·Electrons like their space because their charges repel each other. 10 Practice: Ask these questions every time you have to write an electron configuration ·Lithium: ·find the element on the periodic table ·what is the period number? 2 ·how many shells? ·what is the group number? ·how many valence electrons? ·what subshell(s) does Li have? ·what is the electron configuration? 2 1 1 s 2 1s 2s1 Now it can get annoying to write out all of these Electron Configuration for the elements further down on the periodic table! So fortunately there is a shortcut! Instead of writing it all out, you can first write the name of the "last" Nobel gas in brackets, and then do the Electron Configuration from there. First Draw the Electron Configuration for Zn... Watch this video, it is basic, but really helps break down the steps...while watching the video, think of how YOU could make it better, because that will be your project for the rest of the week!!! http://www.youtube.com/watch?v=JNPFR22MPA