Free Energy review
advertisement

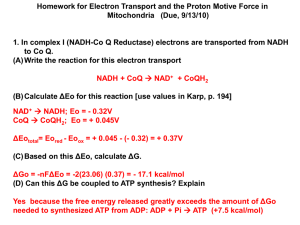
Thermodynamics Chemical reactions proceed according to the rules of thermodynamics • The law of conservation of energy – energy can be converted from one form to another but the total amount of energy is constant • Entropy – the universe is becoming more chaotic ACK! Thermodynamics Some constants Gas constant: R = 8.315 Joules/K* mol or 1.9872 cal/K.mol Faradays constant: F = 96485 Joules/Volt.mol or 23062 cal/Volt* mol Energy: definitions Energy – ability to do work Energetics – energy transfer Types of energy • Potential – trapped energy • Kinetic – energy of movement Energy Categories: more definitions • Radiant energy – energy released from one object to another • Mechanical energy – energy to move objects from place to place • Electrical energy – energy that results from the movement of charged particles down a charge gradient • Thermal energy – reflected in the movement of particles and serves to increase temperature • Chemical energy – energy that is held within chemical bonds Energy Categories, Cont. Animals rely on all five types of energy, which are interconvertible Food Webs are Transfers of Energy Figure 2.3 Thermodynamics in a biological setting Free Energy (G) 1. Change in free Energy (ΔG) ΔG = Products – Reactants ΔG negative – reaction will proceed forward → ΔG positive – reaction will proceed backward ← ΔG zero – reaction at equilibrium ↔ 2. Standard free Energy – ΔGo: 298 K (25oC), 1 atm pressure, pH 7.0 and 1M [initial] for all reactants and products Thermal Energy Thermal energy movement of molecules Most chemical reactions involve changes in thermal energy • Exothermic reactions – release heat • Endothermic reactions – absorb heat Chemical Reactions and Thermal Energy Enthalpy Enthalpy – average thermal energy of a collection of molecules i.e. bond energy Change in enthalpy (DH) = Hproducts – Hsubstrates • Exothermic: DH is negative i.e. C6H12O6 + 6O2 → 6CO2 + 6H2O + energy • Endothermic: DH is positive i.e. ADP + Pi → ATP Chemical Reactions and Thermal Energy Enthalpy and Entropy together Entropy (S) – measure of randomness or disorder Exothermic: DH is negative, increase in DS → reaction will occur spontaneously – negative DG Endothermic: DH is positive, DS is positive → reaction will occur spontaneously. It has to overcome the positive DH Free Energy: calculations Free energy changes of reactions are additive (coupled reactions): Consider the phosphorylation of glucose to glucose 6-phosphate: DGo: glucose + Pi ↔ glucose-6-phosphate + H2O = 3.3 kcal/mol DGo: ATP + H2O ↔ ADP + Pi = -7.3 kcal/mol Summing these reactions together: ATP + glucose ↔ ADP + glucose 6-phosphate DG° = +3.3 + (-7.3) = - 4kcal/mol (favourable) Biological reactions DG = DGo + RTln ([products]/[reactants]) Where R = gas constant, T = temperature in Kelvin Example: glucose + ATP ↔ glucose-6-phosphte + ADP DGo: glucose + Pi ↔ glucose-6-phosphate + H2O = 3.3 kcal/mol DGo: ATP + H2O ↔ ADP + Pi = -7.3 kcal/mol Glucose: [5mM]; ATP: [2mM]; ADP: [0.15mM]; glucose-6phosphate: [0.05mM] So, DG = - 4.0 kcal/mol + 1.9872cal/K mol)(298K)ln((0.05*0.15)/(5*2)) = -8.26kcal/mol ΔG for reactions that don’t make or break bonds DGo is zero - Examples: glucose transport, ion transport across membranes DG = RTln ([inside]/[outside]) Or for charged ions: DG = RTln ([inside]/[outside]) + zFEm where z = valence of the ion; F = Faraday constant and Em = membrane potential Transport across membranes DG = RTln ([inside]/[outside]) + zFEm where z = valence of the ion; F = Faraday constant and Em = membrane potential Example: Diffusion of Cl- from out to in Cl- outside cell: 120mM; Cl- inside cell: 10mM; Em = -80mV DG = (1.987cal/K mol)(298K)(ln(10/120) + (-1)(23062 cal/V mol)(-0.08V) = 376 cal/mol