Homework Answers
advertisement
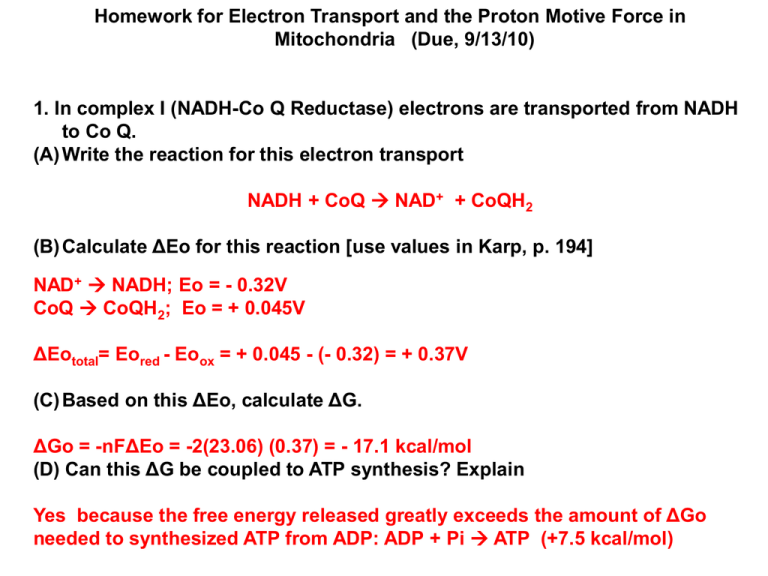
Homework for Electron Transport and the Proton Motive Force in Mitochondria (Due, 9/13/10) 1. In complex I (NADH-Co Q Reductase) electrons are transported from NADH to Co Q. (A) Write the reaction for this electron transport NADH + CoQ NAD+ + CoQH2 (B) Calculate ΔEo for this reaction [use values in Karp, p. 194] NAD+ NADH; Eo = - 0.32V CoQ CoQH2; Eo = + 0.045V ΔEototal= Eored - Eoox = + 0.045 - (- 0.32) = + 0.37V (C) Based on this ΔEo, calculate ΔG. ΔGo = -nFΔEo = -2(23.06) (0.37) = - 17.1 kcal/mol (D) Can this ΔG be coupled to ATP synthesis? Explain Yes because the free energy released greatly exceeds the amount of ΔGo needed to synthesized ATP from ADP: ADP + Pi ATP (+7.5 kcal/mol) 2. In complex IV (cytochrome c oxidase) electrons are transported from cytochrome a3 to O2. (A) Write the reaction for this electron transport ½ O2 + cyt a3red cyt a3ox + H2O (B) Calculate ΔEo for this reaction [use values in Karp, p. 190 or 194] 2 cyt a3ox 2 cyt a3red ; Eo = + 0.385 V ½ O2 H2O; Eo = + 0.816 V ΔEototal = Eored - Eoox = 0.816 - (+ 0.385) = + 0.43 V (C) Based on this ΔEo, calculate ΔG. ΔGo = -nFΔEo = -2(23.06) (0.43) = - 19.8 kcal/mol (D) Can this ΔG be coupled to ATP synthesis? Explain. Yes because the free energy released greatly exceeds the amount of ΔGo needed to synthesized ATP from ADP: ADP + Pi ATP (+ 7.5 kcal/mol) 3. Suppose that two components of an electron transport chain cytochromes x and y have Eo values of + 0.4 and +0.3, respectively. (A) In what direction do electrons flow between these two components? cyt y cyt x (B) What is the Δ Eo for this reaction? ΔEototal = Eored - Eoox = +0.4 - (+0.3) = + 0.10 V (C) Based on this Δ Eo, what is the Δ G for this reaction? ΔGo = -nFΔEo = -2(23.06) (0.10) = - 4.61 kcal/mol (D) Can this ΔG be coupled to ATP synthesis? Explain. No the ΔGo is too small. At least – 7.5 kcal/mol is required 4. (A) Calculate the proton motive force (pmf) across a mitochondrial inner membrane where the electrical potential across the membrane, ψ = -100 mV and the Δ pH = 0.1. Δp = ψ -59 Δ pH = -100 mV - 59(0.1) = -100 - 5.9 = -106 mV = - 0.106 V (B) What is the Δ G for the movement of a proton down this proton generated gradient? ΔGo = zFΔp = (23.06) (- 0.106 V) = - 2.44 kcal/mol (C) How many protons would have to be transported down this proton gradient to generate enough Δ G for the synthesis of ATP from ADP? Explain. Based on a ΔGo of +7.5 kcal/mol for ADP ATP, it would take a minimum of 3 protons and likely 4 or 5 for synthesis of 1 ATP