27.1 Nuclear Reaction (1 Hour)
advertisement
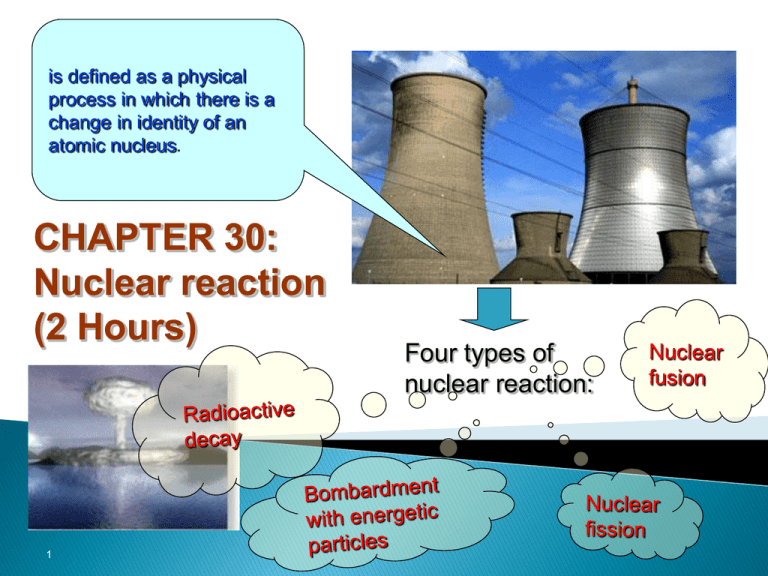
is defined as a physical process in which there is a change in identity of an atomic nucleus. CHAPTER 30: Nuclear reaction (2 Hours) 1 Four types of nuclear reaction: L.O: At the end of this topic, students should be able to: State the conservation of charge (Z) and nucleon number (A) in a nuclear reaction. Write and complete the equation of nuclear reaction. Calculate the energy liberated in the process of nuclear reaction 2 27.1 Nuclear Reaction Definition A nuclear reaction is defined as a physical process in which there is a change in identity of an atomic nucleus. Example 6 3 Li + H 2 H e 2 1 4 2 Figure 27.1 3 27.1.1 Conservation of nuclear reaction In any nuclear reaction, several conservation laws must be obeyed, primarily conservation of charge and conservation of nucleons. – Conservation of charge (atomic number Z): atomic number before reaction Z atomic number after reaction Z – Conservation of mass number A (nucleon) mass number A before reaction mass number after reaction A 4 27.1.2 Reaction Energy, Q Reaction energy is the energy released (liberated) in a nuclear reaction in the form of kinetic energy of the particle emitted, the kinetic energy of the daughter nucleus and the energy of the gamma-ray photon that may accompany the reaction. Reaction energy , Q Δmc M ass d ifferen ce Δm 2 mass of nucleus before reaction mass of nucleus product after reaction m mi mf 5 Note: Δm = mi - mf a) If Δm or Q > 0 (positive value) - exothermic (exoergic) reaction. - energy is released. b) If Δm or Q < 0 (negative value) - endothermic (endoergic) reaction. - energy is required/absorbed in the form of kinetic energy of the bombardment particle. Other reference : Δm = mf – mi Δm →negative (energy is released) Δm →positive (energy is absorbed) 6 27.1.3 Radioactive Decay is defined as the phenomenon in which an unstable nucleus disintegrates to acquire a more stable nucleus without absorb an external energy. The disintegration is spontaneous and most commonly 4 involves the emission of an alpha particle ( OR 2 He ), 0 0 a beta particle ( OR 1 e ) and gamma-ray ( OR 0 γ ). It also release an energy Q known as disintegration energy. Example: 212 84 Po 66 28 208 82 Pb 2 He Q Ni 4 66 29 X 0 1 eQ Ti 208 81 Ti γ 208 81 7 27.1.4 Bombardment with energetic particles is defined as an induced nuclear reaction that does not occur spontaneously; it is caused by a collision between a nucleus and an energetic particles such as proton, neutron, alpha particle or photon. Consider a bombardment reaction in which a target nucleus X is bombarded by a particle x, resulting in a daughter nucleus Y, an emitted particle y and reaction energy Q: X x y Y Q sometimes this reaction is written in the more compact form: X x, y Y daughter target (parent) nucleus nucleus emitted bombarding particle particle 8 Example: 14 7 N 2 He 8 O 1 H Q 4 7 1 Li 3 1H 10 5 17 1 2 2 He Q OR 4 14 7 OR N , p 8 O 17 p , 42 He 10 7 B n , 5 3 Li 7 3 Li 1 7 4 B 0 n 3 Li 2 He Q OR 9 Example 27.1 1. Complete the following radioactive decay equations : Be 2 He 4 a. 8 4 b. 240 Po 97 Sr 139 Ba 94 38 56 c. 236 U 131 I 3 92 53 n 1 0 d. 29 Na 0 e 11 1 40 19 K 20 Ca 40 e. 47 Sc 47 Sc 21 21 f. 10 Example 27.2 When lithium 7Li is bombarded by a proton, two alpha 4He particles are produced. Calculate the reaction energy. Given 1 1 H mass 1.007825 u 7 3 Li mass 7.016003 u 4 2 He mass 4.002603 u Solution: 11 12 Example 27.3 A deuterium bombards a nuclide. 13 6 14 C nuclide and produces 7 N a) Write an equation for the nuclear reaction. b) Calculate the kinetic energy (in MeV) that is released in the reaction. Given : 13 6 2 1 14 7 C mass 13.00335 H mass 2.01410 N mass 14.00307 1 0 n mass 1.00867 u u u u 13 Solution 14 EXERCISE 1. Calculate the energy released in the alpha decay below: 238 92 2. U 234 90 Th 2 He Q (Given mass of U-238 is 238.050786 u ; mass of Th-234 is 234.043583 u and mass of particle is 4.002603 u) ANS. : 6.871013 J The following nuclear reaction is obtained : 14 1 14 1 N n C H 0 . 55 MeV 7 0 6 1 14 6 3. 4 Determine the mass of C in atomic mass unit (u). (Given the mass of nitrogen nucleus is 14.003074 u) ANS. :14.003872 u 7 4 A nuclear reaction can be written as 3 Li p , 2 He . Calculate the energy involved in the reaction and state whether it is absorbed or released. (Given mass of Helium is 4.002602u, mass of Lithium is 7.016003u and mass of proton is 1.006024u). ANS. :15.67MeV 15 L.O: At the end of this topic, students should be able to: Distinguish the processes of nuclear fission and fusion. Explain the occurrence of fission and fusion using the graph of binding energy per nucleon. Explain chain reaction in nuclear fission of a nuclear reactor. Describe the process of nuclear fusion in the sun. 16 27.2.1 Nuclear Fission Definition Nuclear fission is the process by which heavy nuclei are split into two lighter nuclei. 235 92 U 0 n 1 236 92 U 35 Br 85 148 57 La 3 0 n Q 1 Energy is released by the process because the average binding energy per nucleon of the fission products is greater than that of the parent. The energy released is in the form of increased kinetic energy of the product particles (neutrons) and any radiation emitted (gamma ray). 17 Nuclear fission can be divided into two ways of processes : i) spontaneous fission very rarely occur (take very long time) ii) induced fission heavy nucleus is bombarded by a particle : proton, alpha particle and neutron (slow neutrons or thermal neutrons of low energy (about 10-2 eV). 18 For example, consider the bombardment of 235 by 92 U slow neutrons. One of the possible reaction is 235 92 U0n 1 236 92 U 35 Br 85 148 57 La 3 0 n Q 1 Nucleus in the excited state. The reaction can also be represented by the diagram in Figure 27.2 85 35 1 0 Br n 235 92 U 236 92 U 148 57 Figure 27.2 1 0 n 1 0 n 1 0 n La Other possible reactions are: 235 92 U0n 1 235 92 236 92 U 36 Kr U0n 1 89 236 92 144 56 Ba U 38 Sr 94 30 n Q 1 139 54 Xe 30 n Q 1 19 Most of the fission fragments (daughter nuclei) of the uranium-235 have mass numbers from 90 to 100 and from 135 to 145 as shown in Figure 27.3. Figure 27.3 20 21 Binding energy per nucleon as a function of mass number,A Figure 27.4 Binding energy per nucleon (MeV/nucleon) Greatest stability daughter nuclei parent nuclei fission Moving toward more stable nuclei Mass number A 22 Explanation of Figure 27.4 An estimate of the energy released in a fission reaction can be obtained by considering the graph in Figure 27.4. From the figure, the binding energy per nucleon for uranium is about 7.6 MeV/nucleon, but for fission fragment (Z~100), the average binding energy per nucleon is about 8.5 MeV/nucleon. Since the fission fragments are tightly bound, they have less mass. The difference in mass (or energy) between the original uranium nucleus and the fission fragments is about 8.5 -7.6 = 0.9 MeV per nucleon. Since there are 236 nucleons involved in each fission, the total energy released is 0.9 MeV nucleon x 236 nucleons 200 MeV 23 Example 27.4 Calculate the energy released (MeV) in the following fission reaction : 235 1 148 85 1 U n La Br 3 nQ 92 0 57 35 0 Given : 235 92 U mass 235.1 u 1 0 n mass 1.01 u 85 35 148 57 Br mass 84.9 u La mass 148.0 u Solution: 24 Example 27.5 Calculate the energy released when 10 kg of uranium235 undergoes fission according to 235 92 U 0 n 1 141 56 Ba 36 Kr 3 0 n Q 92 1 (Given mass of U-235 is 235.04u, mass of Ba-141 is 140.91u, mass of Kr-92 is 91.91u, mass of neutron is 1.01u and NA=6.02x1023mol-1) Solution: 25 26 27.2.2 Chain Reaction Figure 27.5 Figure 27.6 27 Definition Chain reaction is a series of nuclear fissions whereby some of the neutrons produced by each fission cause additional fissions. Condition to achieve chain reaction in a nuclear reactor a) Slow neutrons are better at causing fission. b) The fissile/fission material must more than a critical size/mass (a few kg). Note: The critical size/mass is defined as the minimum mass of fissile/fission material required to produce a sustained chain reaction. 28 Nuclear Reactor A nuclear reactor is a device in which energy is generated by a controlled fission chain reaction. (The uncontrolled chain reactions are used in nuclear weapons – atomic bomb ) Apart from being used to obtain energy from the reaction of fission, a reactor is widely applied, for example to generate : - radioactive elements, - new fissile materials, such as 233U or 239Pu, - neutrons for scientific research. 29 A nuclear reactor Figure 27.7 30 Figure 27.8 31 • A nuclear reactor consists of fuel rods (fission material), movable control rods and a moderator (water). • Fission reactors use a combination of 235U and 238U (3-5% 235 U). • The 235U will fission, while the 238U(more stable) merely absorbs neutrons (slow neutrons). • Firstly, neutron is bombarded to the 235U and other neutrons are emitted during fission. • Then the emitting neutrons with high energy are slowed down by collisions with nuclei in the surrounding material, called moderator, so that they can cause further fissions and produce more energy. 32 • In order to release energy at a steady rate, the rate of the reaction is controlled by inserting or withdrawing control rods made of elements (often cadmium) whose nuclei absorb neutrons without undergoing any additional reaction. • To have a self-sustaining chain reaction, the mass of fission material must be sufficiently large (> critical mass) so that on the average at least one neutron produced in each fission must go on to produce another fission. 33 27.2.3 Nuclear Fusion Definition Nuclear fusion is the process in which nuclei of light element combine to form nuclei of heavier elements. Examples 2 2 3 H H 1 1 2 He 0n Q 1 Deuterium 2 1 H 2 2 3 1 H H H 1 1 1 1H Tritium 3 1 H Fusion reaction Alpha 4 He 2 particle Q 2 3 4 H H 1 1 2 He 0 n Q 1 Neutron 1 0 Figure 27.9 n 34 The energy released in this reaction is called thermonuclear energy. The amount of energy released by this process can be estimated by using the binding energy per nucleon curve (Figure 27.10). From Figure 27.10, the binding energy per nucleon for the lighter nuclei (2H) is small compared to the heavier nuclei. The energy released per nucleon in the fusion process is given by the difference between two values of binding energy per nucleon. And it is found that the energy released per nucleon by this process is greater than the energy released per nucleon by fission process. 35 Binding energy per nucleon as a function of mass number,A Figure 27.10 Binding energy per nucleon (MeV/nucleon) Greatest stability Moving toward more stable nuclei fusion Mass number A 36 Figure 27.11 37 For two nuclei to undergo fusion, as both nuclei are positively charged there is a strong repulsive force between them, which can only be overcome if the reacting nuclei have very high kinetic energies. These high kinetic energies imply temperatures of the order of 108 K. At these elevated temperatures, however fusion reactions are self sustaining and the reactants are in form of a plasma (i.e. nuclei and free electron) with the nuclei possessing sufficient energy to overcome electrostatic repulsion forces. The nuclear fusion reaction can occur in fusion bomb and in the core of a star. 38 Example 27.6 A fusion reaction is represented by the equation below: 2 2 3 1 H H H 1 1 1 1H Calculate a. the energy in MeV released from this fusion reaction, b. the energy released from fusion of 1.0 kg deuterium, (Given mass of proton =1.007825 u, mass of tritium =3.016049 u and mass of deuterium =2.014102 u) Solution : 39 40 27.2.4 Nuclear Fusion in the sun The sun is a small star which generates energy on its own by means of nuclear fusion in its interior. The fuel of fusion reaction comes from the protons available in the sun. The protons undergo a set of fusion reactions, producing isotopes of hydrogen and also isotopes of helium. However, the helium nuclei themselves undergo nuclear reactions which produce protons again. This means that the protons go through a cycle which is then repeated. Because of this proton-proton cycle, nuclear fusion in the sun can be self sustaining. The set of fusion reactions in the proton-proton cycle can be illustrated by Figure 27.12. 41 positron (beta plus) neutrino 1 1 2 0 H H H 1 1 1 1e vQ 2 1 3 1 H 1 H 2 He 3 2 He Q 2 He 2 He 1 H 1 H Q 3 4 1 1 Figure 27.12 The amount of energy released per cycle is about 25 MeV. Nuclear fusion occurs in the interior of the sun because the temperature of the sun is very high (approximately 1.5 107 K). 42 27.2.5 Comparison between nuclear Fission and nuclear Fusion Differences Fission Heavy to light nucleus Fusion Light to heavy nucleus Neutron to bombard High temperature Produce more than 1 nucleus Produce 1 nucleus Easy to handle & control Difficult to handle & control Similarities new product is produced. energy is released. mass is reduced after reaction. 43 Figure 27.13 Binding energy per nucleon (MeV/nucleon) Greatest stability Fission The falling part of the binding energy curve shows that very heavy elements such as uranium can produce energy by fission of their nuclei to nuclei of smaller mass number. Fusion The rising part of the binding energy curve shows that elements with low mass number can produce energy by fusion. Mass number A 44 45