q rxn
advertisement

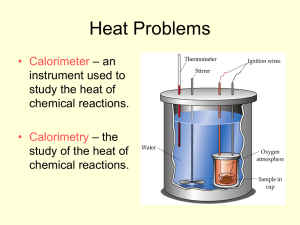
Thermochemistry Part 2: Calorimetry Questions to ponder… If you leave your keys and your chemistry book sitting in the sun on a hot summer day, which one is hotter? Why is there a difference in temperature between the two objects? Because… Different substances have different specific heats (amount of energy needed to raise the temperature of 1 g of a substance by 1 degree Celsius). Questions to ponder… How much heat does it take to melt an ice-cube? Questions to ponder… How much heat does it take to melt an ice-cube? Q=mc∆T, but ∆T=0 Questions to ponder… How much heat does it take to melt an ice-cube? But I KNOW q≠ 0 Questions to ponder… How much heat does it take to melt an ice-cube? How can I solve this??? Calorimetry!!! Heat required to melt ice (a.k.a. latent heat of fusion) cannot be measured directly, but calorimetry provides an experimental method allowing this heat transfer to be measured indirectly. Calorimetry!!! Calorimetry: measurement of the amount of heat evolved or absorbed in a chemical reaction, change of state or formation of a solution. CALORIMETRY The enthalpy change associated with a chemical reaction or process can be determined experimentally. Measure the heat gained or lost during a reaction at CONSTANT pressure A calorimeter is a device used to measure the heat absorbed or released during a chemical or physical process Coffee Cup Calorimeter The cup is filled with water, which absorbs the heat evolved by the reaction, so: qice = -qwater OR qrxn = -qcal Coffee Cup Calorimeter A more “high tech” drawing… Styrofoam cup What happens in a calorimeter? One object will LOSE heat, and the other will ABSORB the heat System loses heat to surroundings = EXO = -q System absorbs heat from surroundings = ENDO = +q When a hot chunk of metal is dropped in a cool glass of water, the metal cools off. Where did the heat from the metal go? Did the metal lose more heat then the water gained? Magnitude of HEAT GAINED = HEAT LOST (ALWAYS!) The numbers in these two boxes are always the same, but with different signs (+/-). What heat one lost, the other gained. To do calorimetry problems… Make a chart: measurement Heat (q) Mass (m) Specific Heat (c) Final Temp (Tf ) Initial Temp(Ti) “Cal” (often water) Object/”Rxn” EXAMPLE 1: A small pebble is heated and placed in a foam cup calorimeter containing 25.0 g of water at 25.0 C. The water reaches a maximum temperature of 26.4 C. How many joules of heat were released by the pebble? The specific heat of water is 4.184 J/g C. Water (cal) Heat Mass 25.0 g Specific Heat 4.184 Final Temp 26.4 oC Initial Temp 25.0 oC Pebble (rxn) The numbers in these two boxes are always the same, but with different signs (+/-). What heat one lost, the other gained. Hints: The pebble lost heat because the water heated up from 25.0 C to 26.4 C. Pebble loses heat (-q, exothermic) while water gains heat (+q, endothermic) Do you calculation based on water (since the problem gave all the water’s information) Water (cal) Pebble (rxn) Heat Mass 25.0 g Specific Heat 4.184 Final Temp 26.4 oC Initial Temp 25.0 oC qcal = mwatercwaterTwater qcal = (25.0g)(4.184J/goC)(26.4oC-25.0oC) qcal = 150 J qrxn = - 150 J If the water ABSORBED 150 J of heat, then the pebble RELEASED 150 J of heat. Example 2: When 1.00 g of ammonium nitrate, NH4NO3, is added to 50.0 g of water in a coffee cup calorimeter, it dissolves, NH4NO3 (s) NH4+(aq) + NO3(aq), and the temperature of the water drops from 25.00C to 23.32C. Calculate q for the reaction system. cal rxn 50.0 g 1.00 g q m c 4.184 Tf 23.32 oC Ti 25.00 oC qcal = mwcwt qcal = (50.0g)(4.18 J/gC)(-1.68C) qcal = - 351 J qrxn = - qcal qrxn = 351 J (endothermic) When one substance absorbs heat, the other substance releases heat (energy) Example 3 (similar to what you will do in tomorrow’s lab) Suppose that 100.00 g of water at 22.4 °C is placed in a calorimeter. A 75.25 g sample of Al is removed from boiling water at a temperature of 99.3 °C and quickly placed in a calorimeter. The substances reach a final temperature of 32.9 °C . Determine the SPECIFIC HEAT of the metal. The specific heat of water is 4.184 J/gC. MAKE YOUR CHART Example 3: Suppose that 100.00 g of water at 22.4 °C is placed in a calorimeter. A 75.25 g sample of Al is removed from boiling water at a temperature of 99.3 °C and quickly placed in a calorimeter. The substances reach a final temperature of 32.9 °C . Determine the SPECIFIC HEAT of the metal. The specific heat of water is 4.184 J/g C. cal (H2O) rxn (Al) 2. Calculate q for water q m c 100.00 g 1. Make chart 75.25 g 3. Q for Al is the same (but with different sign) as q for metal. 4. Using q metal, calculate c metal 4.184 Tf 32.9 oC 32.9 oC Ti 22.4 oC 99.3 oC Example 3 cont’d: specific heat of aluminum… cal (H2O) rxn (Al) qcal = mwcwT qcal = (100.00g)(4.184J/gC)(10.5C) q 4,393.2 J m 100.00 g c 4.184 J/gC Tf 32.9 C 32.9 C qrxn = - qcal Ti 22.4 C 99.3 C qrxn = -4,393.2 J -4,393.2 J 75.25 g qcal = 4,393.2 J qrxn = mAlcAlT -4,393.2 J = (75.25 g)(cAl)(-66.4C) cAl = 0.879 J/gC What if you wanted to measure the heat of a reaction or process that couldn’t be measured in a simple coffee cup calorimeter (e.g., heat of combustion of Mg)? You would need something like this… Bomb Calorimeter Another type of calorimeter is a Bomb Calorimeter NOTE: In a bomb calorimeter, heat is transferred from the sample to the oxygen-enriched chamber, to the metal that makes up the chamber, to the water… thus we cannot just use the specific heat of water; instead heat capacity of the calorimeter, Ccal, can be used or calculated. If Heat Capacity ( C) is known It is possible to calculate the amount of heat absorbed or evolved by the reaction if you know the heat capacity, Ccal, and the temp. change, ΔT, of the calorimeter: qcal = C ΔT cal Everything else is the same (remember, the heat lost from the reaction goes into the calorimeter) EXAMPLE 4: The reaction between hydrogen and chlorine, H2 + Cl2 2HCl, can be studied in a bomb calorimeter. It is found that when a 1.00 g sample of H2 reacts completely, the temp. rises from 20.00C to 29.82C. Taking the heat capacity of the calorimeter to be 9.33 kJ/C, calculate the amount of heat evolved in the reaction. Cal q c 9.33 kJ/oC Tf 29.82 oC Ti 20.00 oC Rxn qcal = CcalΔT qcal = (9.33 kJ/C)(9.82 C) qcal = 91.6 kJ qrxn = - 91.6 kJ *** must have a negative sign if “heat evolved in the reaction” = released EXAMPLE 5: When 1.00 mol of caffeine (C8H10N4O2) is burned in air, 4.96 x 103 kJ of heat is evolved. Five grams of caffeine is burned in a bomb calorimeter. The temperature is observed to increase by 11.37C. What is the heat capacity of the calorimeter in J/C? 4.96 x 103 kJ is for 1.00 mol of caffeine. We are burning only 5.00 g of caffeine to increase the temp by 11.37 oC. We FIRST need to figure out how much heat energy is given off by just 5 grams. 4.96 10 3 kJ x 194.0g C 8H10N4 O2 5.00 g Cal q +128 kJ c ? T 11.37 oC Rxn x 128 kJ 128kJ (Ccal ) (11.37C) -128 kJ C cal 11.3 kJ C 11,300 J C EXAMPLE 6: When twenty milliliters of ethyl ether, C4H10O. (d=0.714 g/mL) is burned in a bomb calorimeter, the temperature rises from 24.7C to 88.9C. The calorimeter heat capacity is 10.34 kJ/C. (a) What is q for the calorimeter? (b) What is q when 20.0 mL of ether is burned? (c) What is q for the combustion of one mole of ethyl ether? Cal q c 10.34 kJ/oC Tf 88.9 oC Ti 24.7 oC Rxn qcal = CcalΔt qcal = (10.34 kJ/C)(64.2 C) qcal = 664 kJ EXAMPLE 6: When twenty milliliters of ethyl ether, C4H10O. (d=0.714 g/mL) is burned in a bomb calorimeter, the temperature rises from 24.7C to 88.9C. The calorimeter heat capacity is 10.34 kJ/C. (a) What is q for the calorimeter? (b) What is q when 20.0 mL of ether is burned? (c) What is q for the combustion of one mole of ethyl ether? qrxn = -qcal qrxn = -664 kJ EXAMPLE 6: When twenty milliliters of ethyl ether, C4H10O. (d=0.714 g/mL) is burned in a bomb calorimeter, the temperature rises from 24.7C to 88.9C. The calorimeter heat capacity is 10.34 kJ/C. (a) What is q for the calorimeter? (b) What is q when 20.0 mL of ether is burned? (c) What is q for the combustion of one mole of ethyl ether? 664kJ 1 mL 74.0 g 3 kJ 3.44 10 mol 20.0 mL 0.714 g 1 mol