Common Structures
advertisement

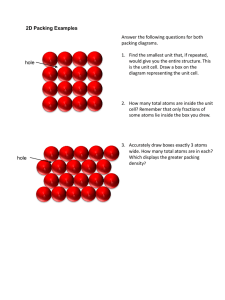
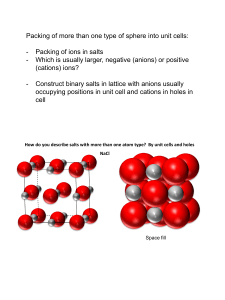
Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, Chemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 e CHEM1002 [Part 2] Dr Michela Simone Weeks 8 – 13 Office Hours: Monday 3-5, Friday 4-5 Room: 412A (or 416) Phone: 93512830 e-mail: michela.simone@sydney.edu.au Slide 2/20 e Summary of Last Lecture Crystal structures I • • • • • Hexagonal close packing arises when close packed layers repeat every third layer (ABABABAB) Cubic close packing arises when close packed layers repeat every fourth layer (ABCABCABC) The coordination number in both types of close packing is 12 Unit cells are the simplest building blocks from which the whole solid can be built Atoms on corners of unit cells are shared between 8 cells, atoms on faces of unit cells are shared between 2 cells, atoms on edges of unit cells are shared between 4 cells and atoms at the centres of unit cells are unshared. Slide 3/20 e Crystal Structures II Lecture 9: • Packing efficiency • Octahedral and tetrahedral interstitial holes • Ionic crystal structures • Blackman Chapter 7, Section 7.4 (pages 265-268) Next week’s lectures • Lectures 10 and 11: solubility • Blackman Chapter 10, Sections 10.1 – 10.4 • Revise equilibrium calculations from CHEM1001! • Lecture 12: introduction to coordination chemistry • Blackman Chapter 13, Sections 13.1 – 13.4 Slide 4/20 x • Packing Efficiency I: Atoms in Cell The unit cell for CCP is face centred cubic (FCC) atoms on each corner and atoms on each face of the cube • Atoms on corners: • Atoms on faces: • Total: Slide 5/20 x • Packing Efficiency II: Volume Occupied by Atoms The unit cell for CCP is face centred cubic (FCC) atoms on each corner and atoms on each face of the cube • If each atom has radius r, volume of atom = • Number of atoms = • Volume occupied by atoms = Slide 6/20 x • Packing Efficiency III: Volume of Unit Cell The unit cell for CCP is face centred cubic (FCC) atoms on each corner and atoms on each face of the cube • Close packed direction is diagonal of face a • Length of diagonal: r a r • Length of side: r r • Volume of cubic unit cell: Slide 7/20 x • Packing Efficiency IV: FCC The unit cell for CCP is face centred cubic (FCC) atoms on each corner and atoms on each face of the cube • Volume occupied by atoms • Volume of unit cell • Packing efficiency Slide 8/20 e Cubic Close Packing: CCP and HCP • Cubic close packing: Layers repeat ABCABCABC Number of atoms in unit cell = 4 Packing efficiency = 74% Coordination number = 12 • Hexagonal close packing Layers repeat ABABAB Packing efficiency = 74% Coordination number = 12 e Other Metal Structures • Simple cubic Number of atoms in unit cell = 1 Packing efficiency = 52% Coordination number = 6 • Body centred cubic Number of atoms in unit cell = 2 Packing efficiency = 68% Coordination number = 8 e Interstitial Holes - Oh • Even in the close packed structures, there are spaces for extra atoms • Octahedral interstitial holes at the centre of the cube and on each edge Slide 11/20 e Interstitial Holes - Td • Even in the close packed structures, there are spaces for extra atoms • Tetrahedral interstitial holes in the centre of the cube corners Slide 12/20 e Common Structures • For every n close packed atoms, there are n octahedral holes and 2n tetrahedral holes • Almost all of the common structures can be thought as of being derived from Close packed (CCP or HCP) anions Cations in octahedral and/or tetrahedral interstitials Slide 13/20 i Common Structures - Rocksalt • NaCl (rocksalt) CCP Cl- anions with Na+ in all of the octahedral interstitials Slide 14/20 i Common Structures - Zinc Blende • Zinc Blende (ZnS) CCP S2- anions with Zn2+ in half of the tetrahedral interstitials Slide 15/20 i Common Structures - Anti-Fluorite • Anti-Fluorite (Na2O) CCP O2- anions with Na+ in all of the tetrahedral interstitials Slide 16/20 i Common Structures - Fluorite • Fluorite (CaF2) CCP Ca2+ cations with F- in all of the tetrahedral interstitials Slide 17/20 i Common Structures Anion Packing ccp hcp Filling of Interstitial Sites Structure Examples Td Oh - all rock-salt NaCl, MgO, LaN - 1/2 cadmium chloride CdCl2, NiCl2 half - zinc blende (sphalerite) ZnS, CuCl, BN all - anti-fluorite Na2O, Li2S 1/4 1/2 spinel MgAl2O4 - all nickel arsenide NiAs, FeS - 1/2 cadmium iodide CdI2, Mg(OH)2 all - wurtzite ZnS, BeO - 1/2 rutile TiO2, CrO2 Slide 18/20 e Summary: Crystal Structures II Learning Outcomes - you should now be able to: • • • • • Complete the worksheet Work out the number of atoms in a unit cell Understand the calculation of packing efficiency Recall the packing efficiency and coordination numbers of HCP, CCP, BCP and simple cubic structures Be able to rationalize ionic structures in terms of filling of interstitial holes Next lecture: • Solubility Slide 19/20 x Practice Examples 1. How many atoms are there in the body-centred cubic unit cell of tungsten? A. # atoms = 1 B. # atoms = 1 + 1/8 × (8) = 2 C. # atoms = 1/2 × (6) = 3 D. # atoms = 1/2 × (6) + 1/8 × (8) = 4 E. # atoms = 1 + 1/2 × (6) + 1/8 × (8) = 5 2. Verify that the efficiency of simple cubic packing is 52% and BCP is 68%. Slide 20/20