Here - OSU Chemistry
advertisement

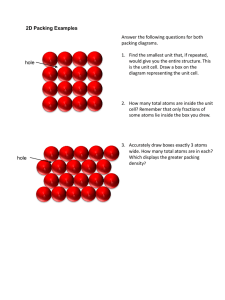
Close Packed Structures (a) Hexagonal and Cubic Close Packing The structures of many metals can be described as close packed arrays of spherical atoms. There are two alternative ways to maximize the packing efficiency of a collection of equally sized spheres. Cubic Close Packing (CCP) ABCABC Hexagonal Close Packing (HCP) - ABABAB Fm3m P63/mmc The figure below illustrates the two packing sequences. The spheres of the second layer can be centered over either the sites marked b or c. The symmetry is exactly the same regardless of the choice, however, when a third layer is added it now has a choice of packing over the spheres in the original layer or over the sites marked as c in the diagram of the first layer. The former option leads to the ABAB... stacking characteristic of hexagonal close packing, while the latter option leads to the ABCABC... packing which defines a cubic close packed structure. The packing efficiency (volume of space occupied by the spheres/total volume) is 74.05 % for both cubic and hexagonal close packing schemes. The number of (12) and distance between nearest neighbors is also identical for both HCP and CCP, but the second nearest neighbor coordination is different. In addition to these two schemes a fair number of metals adopt a body centered cubic arrangement. In this structure type the number of nearest neighbors has been reduced from 12 to 8. This does not result in a close packed array of spheres, and the packing efficiency drops to 68.02%. (b) Crystallographic Unit Cells Now that we are familiar with cubic and hexagonal close packing, let us turn to their crystallographic descriptions. Cubic close packing leads to a structure with a face centered cubic unit cell as shown below. For this reason cubic close packing is sometimes called face centered cubic (fcc) packing. This structure represents the simplest of all structures based on a face centered cubic Bravais lattice, one with a single atom as the basis (asymmetric unit). Space Group = Fm3m Composition = M Atom M site x 4a 0 y 0 z 0 It is not immediately obvious how the fcc unit cell is related to ccp stacking described in the previous section. To see the relationship begin by finding the 3-fold rotation axes perpendicular to the layers in the ABC… stacking scheme. They should be fairly easy to spot. Next consider that the 3-fold axes run along the body diagonal in a cubic unit cell. Combining these two we see that the layers from the ccp stacking are perpendicular to the body diagonal of the fcc unit cell. This relationship is highlighted in the figure below where the atoms in layer A are shaded green, those in layer B are blue, and those in layer C are red. The unit cell and crystallographic description corresponding to hexagonal close packing are shown below. Space Group = P63/mmc Composition = M Atom M site x 2a 0 y 0 z 0 Here the relationship between the crystallographic unit cell and the layer stacking is easily seen (atoms in layer A are shaded blue and those in layer B are shaded red in the figures above). The atoms in layer A are located at the vertices of the hexagonal unit cell, while one atom from layer B is contained in each unit cell at 1/3, 2/3, 1/2. (c) Body Centered Cubic Packing Another highly symmetric packing arrangement, sometimes, adopted by metals, is the body centered cubic structure shown below. Space Group = Im3m Composition = M Atom M Site x 2a 0 y 0 z 0 Unlike ccp and hcp arrangements each atom in the bcc arrangement has 8 nearest neighbors (rather than 12). Consequently, the packing efficiency is lower (as we shall see in the next section) and body centered cubic packing cannot be considered a close packed arrangement. (d) Packing Efficiency and Theoretical Density The packing efficiency of a structure can be calculated if we assume the atoms are hard spheres. In that case the packing efficiency is given by = (volume occupied by spheres) / (total volume) This calculation is carried out on the contents of the unit cell. Therefore, our first step is to count the number of atoms contained within the unit cell. To do this you should keep in mind that atoms located at the corners, edges and faces of the unit cell are not fully contained in a single unit cell, so that they only partially occupy the unit cell. Atom Location Corner Edge Face Anywhere else Fraction Inside Unit Cell* 1/8 1/4 1/2 1 *For unit cells with non-orthogonal axes it is not strictly true that an atom on a corner (edge) is exactly 1/8 (1/4) inside the unit cell, but it is still true that 8 (4) such atoms add up to one atom in the unit cell. Now we can determine the number of atoms inside the unit cell for each of the packing arrangements we have discussed. Face Centered Cubic (ccp) (8 ×1/8) + (6 ×1/2) = 4 Body Centered Cubic (bcc) (8 ×1/8) + 1 = 2 The volume of occupied space contained within the unit cell can now be easily calculated by multiplying the number of atoms per unit cell by the volume of a sphere. V = 4/3 r3 The next step is to determine the size of the unit cell as a function of the atomic radius, r: Face Centered Cubic 4r = (2)1/2/a Body Centered Cubic 4r = (3)1/2/a Face Centered Cubic volume = a3 = [4r/(2)1/2]3 = 16(2)1/2 r3 Body Centered Cubic volume = a3 = [4r/(3)1/2]3 = 64/[3(3)1/2]r3 Now we can easily calculate the packing efficiency: Face Centered Cubic 4[4/3 r3] / [16(2)1/2 r3] = / (3(2)1/2) = 0.7405 = 74.05% Body Centered Cubic 2[4/3 r3] / [64/(3(3)1/2)r3] = (3)1/2/8 = 0.6802 = 68.02% As promised the packing efficiency of the bcc structure is lower than the fcc structure. With a little bit more work it can be shown that the hcp structure has the exact same packing density as the fcc arrangement. From this point with a relatively easy extension of our methods, it is possible to calculate the theoretical density of a compound from its structure. Since density is a mass per unit volume we need to calculate the total atomic weight of all atoms in the unit cell then divide by the volume of the unit cell. Example Silver crystallizes with a fcc structure and a lattice parameter a=4.086 Å. What is its theoretical density? Density = mass/volume = 4 (107.87 g/mol)(1 mol/6.0221023 atoms)/ (4.08610-8 cm)3 = 10.50 g/cm3 (e) Body Centered Cubic Packing Since the number and arrangement of nearest neighbors are identical in ccp and hcp, it is reasonable to expect that the free energies of these two structures would be very similar. Thus it should not come as a surprise that both structure types (along with bcc) are commonly observed. Furthermore, the addition of small levels of impurities can lead to a change in the structure type. This phenomena has important technological implications in several areas, including making steel from iron. Cubic close packed, a Cu 3.6147 Ag 4.0857 Au 4.0783 Al 4.0495 Ni 3.5240 Pd 3.8907 Pt 3.9239 Pb 4.9502 (f) Hexagonal close packed, a, c Be 2.2856, 3.5832 Mg 3.2094, 5.2105 Zn 2.6649, 4.9468 Cd 2.9788, 5.6167 Ti 2.506, 4.6788 Zr 3.312, 5.1477 Ru 2.7058, 4.2816 Os 2.7353, 4.3191 Re 2.760, 4.458 Body centered cubic, a Fe 2.8664 Cr 2.8846 Mo 3.1469 W 3.1650 Ta 3.3026 Ba 5.019 Ordered Structures Several structures can be described as ordered derivatives of the fcc, bcc and hcp structures. Examples of this include the CsCl structure, which is an ordered derivative of the bcc structure, as well as the Cu3Au and CuAu structures, which are ordered derivatives of the fcc structure. Note that in each of these structures the symmetry is lower than in the parent structure. This is a consequence of the fact that two atoms which would be equivalent in an elemental compound, are no longer equivalent in an ordered compound (i.e., the atoms at 0,0,0 and ½, ½, ½ in CsCl). Thus the symmetry operation which related these two atoms in the parent structure (i.e., body centering translation) is destroyed, and a reduction in symmetry results.