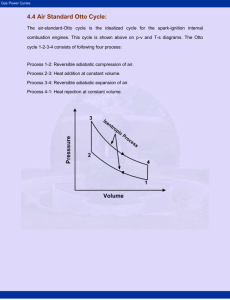
Air-Standard Cycles and Their Analysis
advertisement
KARADENİZ TECHNICAL UNIVERSITY İsmail ALTIN, PhD Assistant Professor Karadeniz Technical University Faculty of Marine Sciences Department of Naval Architecture and Marine Engineering TURKEY Research interest • Internal combustion engines • Thermodynamic modeling of ICEs • Fuels and combustion For details: www.ismailaltin.info Air-Standard Cycles and Their Analysis Contents 1. 2. 3. 4. 5. 6. 7. Introduction Air-standart cycle Analysis of Dual cycle Analysis of Otto cycle Analysis of Diesel cycle Comparison of the cycles Comprehensive examples 1.Introduction In internal combustion engines (ICE), the conversion of heat energy into mechanical work is a complicated process. To examine all these changes quantitatively and to account for all the variables, creates a very complex problem. 1.Introduction… The two commonly employed approximations of an actual engine in order of their increasing accuracy are (a) the air-standard cycle and (b) the fuel-air cycle. They give an insight into some of the important parameters performance. that influence engine 1.Introduction… In the air-standard cycle the working fluid is assumed to be air. The values of the specific heat of air are assumed to be constant at all temperatures. This ideal cycle represents the upper limit of the performance, which an engine may theoretically attain. 2. Air-standart cycle The analysis of the air-standard cycle is based on the following assumptions: 1. The working fluid in the engine is always an ideal gas, namely pure air with constant specific heats. 2. A fixed mass of air is taken as the working fluid throughout the entire cycle. The cycle is considered closed with the same air remaining in the cylinder to repeat the cycle. The intake and exhaust processes are not considered. 3. The combustion process is replaced by a heat transfer process from an external source. 4. The cycle is completed by heat rejection to the surrounding until the air temperature and pressure correspond to initial conditions. This is in contrast to the exhaust and intake processes in an actual engine. 2. Air-standart cycle… 5. All the processes that constitute the cycle are reversible. 6. The compression and expansion processes are reversible adiabatic. 7. The working medium does not undergo any chemical change throughout the cycle. 8. The operation of the engine is frictionless. Because of the above simplified assumptions, the peak temperature, the pressure, the work output, and the thermal efficiency calculated by the analysis of an air-standard cycle are higher than those found in an actual engine. However, the analysis shows the relative effects of the principal variables, such as compression ratio, inlet pressure, inlet temperature, etc. on the engine performance. 2. Air-standart cycle… In this lecture, the following air-standard cycles are described and their work output, thermal efficiency, and mean effective pressure are evaluated: 1. Otto cycle 2. Diesel cycle 3. Dual cycle Some shortcomings of these ideal cycles are obvious, but these cycles give a valuable insight into real effects and possibilities. 3. Analysis of Dual cycle It is a theoretical cycle for modern high speed diesel engines. The heat supplied is partly at constant volume and partly at constant pressure. This cycle is also called the mixed cycle or limited pressure cycle. The compression and expansion processes are isentropic and heat is rejected at constant volume. The p-V and T-s diagrams are shown in Figures 3.1 (a) and (b) respectively. 3. Analysis of Dual cycle… pV const. dQ 0 Figure 3.1. Dual cycle Here: Process 1-2 is isentropic compression. Process 2-3 is reversible constant volume process. Process 3-4 is reversible constant pressure process. Process 4-5 is isentropic expansion. Process 5-1 is reversible constant volume process. 3. Analysis of Dual cycle… Heat supplied during the process 2-3 mcv (T3 T2 ) Heat supplied during the process 3-4 mc p (T4 T3 ) Total heat supplied, Q1 mcv (T3 T2 ) mc p (T4 T3 ) Total rejected during process 5-1, Q2 mcv (T5 T1 ) (3.1) (3.2) Thermal efficiency: (3.3) 3. Analysis of Dual cycle… Three ratios are used to analysis the Dual cycle: Q1 (3.4) Q2 (3.5) (3.6) Three ratios are always greater than 1. 3. Analysis of Dual cycle… (3.7) (3.8) (3.9) 3. Analysis of Dual cycle… (3.10) 3. Analysis of Dual cycle… Substituting the values of T1, T2, T3 from Eqs. (3.7), (3.8), (3.9) and (3.10) respectively in Eq. (3.3), (3.11) 3. Analysis of Dual cycle… Equation (3.11) shows that the increase in the compression ratio r, and the higher values of the adiabatic exponent cause an increase in the thermal efficiency. With a constant amount of heat added, the values of α and β depend on what part of the heat is added at constant volume and what part at constant pressure. An increase in the value of α and the corresponding reduction in β results in a higher thermal efficiency. 3. Analysis of Dual cycle… Working done during cycle, (3.12) 3. Analysis of Dual cycle… Swept volume, (3.13) Mean effective pressure, (3.14) (3.15) 4. Analysis of Otto cycle A German scientist, A. Nicolaus Otto in 1876 proposed an ideal air-standard cycle with constant volume heat addition, which formed the basis for the practical spark-ignition engines (petrol and gas engines). The cycle is shown on p-V and T-s diagrams in Figure 4.1(a) and Figure 4.1(b) respectively. 4. Analysis of Otto cycle… pV const. dQ 0 Q1 Q2 Figure 4.1. Otto cycle Here: Process 1-2 is isentropic compression. Process 2-3 is reversible constant volume process. Process 3-4 is isentropic expansion. Process 4-1 is reversible constant volume process. 4. Analysis of Otto cycle… For dual cycle, thermal efficiency has been defined as 1 1 1 r 1 1 V3 1 is used for thermal efficiency of Otto cycle. We get V2 1 1 r 1 (3.16) 4. Analysis of Otto cycle… Figure 4.2. Thermal efficiency vs. compression ratio for different values of the adiabatic exponent 4. Analysis of Otto cycle… Mean effective pressure, p1r pm 1 1 1 r 1 1 p1 1 r pm 1 r 1 (3.17) Figure 4.3 Mean effective pressure vs. pressure ratio for different values of compression ratio r. 5. Analysis of Diesel cycle Q1 pV const. dQ 0 Q2 Figure 5.1. Diesel cycle Here: Process 1-2 is isentropic compression. Process 2-3 is reversible constant pressure process. Process 3-4 is isentropic expansion. Process 4-1 is reversible constant volume process. 5. Analysis of Diesel cycle… For dual cycle, thermal efficiency has been defined as 1 1 1 r 1 1 p3 1 is used for thermal efficiency of Diesel cycle. We get p2 1 1 1 1 r 1 (3.18) 5. Analysis of Diesel cycle… Figure 5.2 Thermal efficiency vs. cut-off ratio at different compression ratios and adiabatic exponents. 5. Analysis of Diesel cycle… Mean effective pressure, p1r pm 1 1 1 r 1 1 p1r pm 1 1 r 1 (3.19) 6. Comparison of the cycles The significant parameters in cycle analysis are compression ratio, peak pressure, peak temperature, heat addition, heat rejection, and the net work. In order to compare the performance of these cycles, some of the parameters are kept fixed. 6. Comparison of the cycles… 6.1. For the same compression ratio and heat addition Figure 6.1. p-V and T-s diagrams having the same compression ratio and heat addition for the three cycles. Otto Dual Diesel 6. Comparison of the cycles… 6.2. For the same compression ratio and heat rejection Figure 6.2. p-V and T-s diagrams having the same compression ratio and heat rejection for the three cycles. Otto Dual Diesel 6. Comparison of the cycles… 6.3. For the same same peak pressure, peak temperature and heat rejection Figure 6.3. p-V and T-s diagrams having the same peak pressure, peak temperature and heat rejection for the three cycles. Diesel Dual Otto 6. Comparison of the cycles… 6.4. For the same maximum pressure and heat input Figure 6.4. p-V and T-s diagrams having the same maximum pressure and heat input for the three cycles. Diesel Dual Otto (for the same, Q1 ) 6. Comparison of the cycles… 6.6. For the same maximum pressure and work output Figure 6.7. T-s diagrams having the same maximum pressure and heat input for the three cycles. Diesel Dual Otto 7. Comprehensive examples Examples of thermodynamic cycles can be found in handout. Reference 1. H.N. Gupta, Fundamentals of Internal Combustion Engines, PHI Learning Private Ltd., New Delhi, 2011. 2. W.W. Pulkrabek, Engineering Fundamentals of The Internal Combustion Engine, Prentice Hall, New Jersey, 2003. FACULTY OF MARINE SCIENCES FACULTY OF MARINE SCIENCES Thank you for your attention QUESTIONS