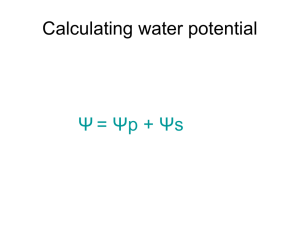Solutions
advertisement

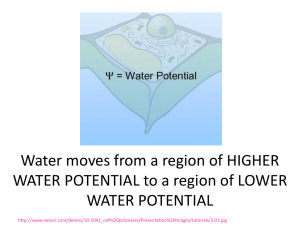
Mixtures and Solutions Chapter 14 Heterogeneous Mixtures Suspensions – Mixture containing particles that settle out if left undisturbed. – Particles are much larger than atoms. Colloids – Heterogeneous mixture of intermediate size particles Particles are smaller than the size of suspension particles and larger than the size of solution particles. – Particles have erratic movement. (Brownian motion) – Particles are large enough to scatter light. (Tyndall effect) Lights shown through fog Solutions What are solutions? Homogeneous mixtures containing two or more substances called the solute (substance that dissolves) and the solvent (dissolving medium). – May exist as gas, liquid, or solid depending on the state of its solvent Solutions Soluble – Substance that dissolves in a solvent (sugar in water) Insoluble – Substance that does NOT dissolve in a solvent (sand in water) Miscible – Two liquids that are soluble in one another (Ethylene glycol and water: anitfreeze) Immiscible – Two liquids that are NOT soluble in one another (oil and vinegar) Solution Concentration Concentration: A measure of how much solute is dissolved in a specific amount of solvent or solution. Concentration Ratios – Percent by mass (Mass of solute/mass of solution) x 100 – Percent by volume (volume of solute/volume of solution) x 100 – Molarity Moles of solute/liter of solution – Molality Mole of solute/kilogram of solvent – Mole Fraction Moles of solute/(moles of solute + moles of solvent) Percent by Mass Percent by mass = (mass of solute/mass of solution) x 100 Example: What is the percent by mass of NaHCO3 in a solution containing 20g NaHCO3 dissolved in 600g H2O? Percent by Volume Percent by Volume = (volume of solute/volume of solution) x 100 Example: What is the percent by volume of ethanol in a solution that contains 35mL of ethanol dissolved in 115mL of water? Molarity Molarity (M) – The number of moles of solute dissolved per liter of solution. – The unit M is read as molar. 1M solution – M = moles of solute/liters of solution Example: What is the molarity of an aqueous solution containing 40.0g of glucose (C6H12O6) in 1.5L of solution? Molar Solutions Examples: – How many grams of CaCl2 would be dissolved in 1.0L of a 0.10M solution of CaCl2? – A liter of 2M NaOH solution contains how many grams of NaOH? Diluting Solutions When diluting from a stock solution to attain different concentrations use the equation: M1V1 = M2V2 Example: What volume of a 3.00M KI solution would you use to make 0.300L of a 1.25M KI solution? Molality the ratio of the number of moles of solute dissolved in one kilogram of solvent. m = moles of solute/kilogram of solvent = moles of solute/1000g of solvent Example: What is the molality of a solution containing 10.0g Na2SO4 dissolved in 1000.0g of water? Mole Fraction The ratio of the number of moles of solute in solution to the total number of moles of solute and solvent. Mole fraction is represented by X. XA = nA/(nA + nB) XB = nB/(nA + nB) Example: What is the mole fraction of NaOH in an aqueous solution that contains 22.8% NaOH by mass? Solvation The process of surrounding solute particles with solvent particles to form a solution. Example: Salt Water – Water particles surround each sodium ion and chlorine ion in the solution – Solvation in water is called hydration. 3 ways to increase rate of solvation. – Agitate the mixture – Increase the surface area of the solute – Increase the temp. of the solvent Heat of solution – overall energy change that occurs during the solution formation process. Solubility Maximum amount of solute that will dissolve in a given amount of solvent. – Usually expressed in grams of solute per 100g of solvent. Saturation – Saturated solution Max. amount of dissolved solute for a given amount of solvent at a given temp. – Unsaturated solution Less dissolved – Supersaturated Contains more dissolved solute than a saturated solution at the same temperature. – Many substances are more soluble at high temperatures than lower temperatures. Increasing the temperature will allow for more solute to dissolve in the same amount of solvent. Solubility Pressure – The solubility of a gas in any solvent increases as its external pressure increases. – Example: Carbonated Beverages Carbon dioxide is dissolved in the solution at a pressure higher than atmospheric pressure. Henry’s Law At a given temperature, the solubility (S) of a gas in a liquid is directly proportional to the pressure (P) of the gas above the liquid. S1/P1 = S2/P2 – Example: If 0.55g of a gas dissolves in 1.0L of water at 20.0kPa of pressure, how much will dissolve at 110.0kPa of pressure? Colligative Properties Physical Properties of solutions that are affected by the number of particles but not the identity of dissolved solute particles. – Vapor Pressure Lowering – Boiling point Elevation – Freezing Point Depression – Osmosis – Osmotic Pressure Vapor Pressure Lowering Due to the number of solute particles in solution and is a colligative property of solutions. The greater number of solute particles in a solution, the lower the resulting vapor pressure. Boiling Point Elevation The temperature difference between a solution’s boiling point and a pure solvent’s boiling point. ∆Tb = Kbm What is the boiling point of a 0.029m aqueous solution of sodium chloride (NaCl)? Freezing Point Depression The difference in temperature between the freezing point of a solution and the freezing point of its pure solvent. ∆Tf = Kfm Example: What is the freezing point of a 0.29m aqueous solution of sodium chloride (NaCl)? Osmosis and Osmotic Pressure Osmosis is the diffusion of solvent particles across a semipermeable membrane from an area of higher solvent concentration to an area of lower solvent concentration. – Semipermeable membranes are barriers with tiny pores that allow some but not all kinds of particles to cross. – Example: kidney dialysis, uptake of nutrients by plants. Osmotic Pressure is the amount of additional pressure caused by the water molecules that moved into the solution. – Dependent upon the number of solute particles in a given volume of solution.