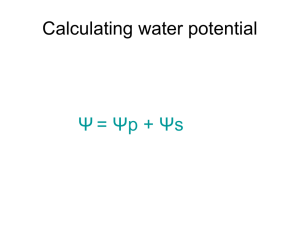Grade 7 Mixtures and Solutions
advertisement

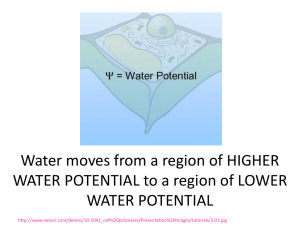
Grade 7 Mixtures and Solutions Review Chapter 4 Matter: Anything that has mass and takes up space Properties: describe matter Mixture: a substance made up of two or more other substances Heterogenous: a substance that looks like it is made up of more than one substance Homogenous: a substance that looks like it is only made up of one susbtance Mehanical Mixture: a mixture where all of the parts that make it up are clearly visible Pure substance: a substance that is made up of all of the same particles: not mixed in any way Particle Theory of Matter: Describes how the particles of substances will look and behave in certain states of matter Chapter 5 Solute: the substance that is being dissolved Solvent: The substance that is doing the dissolving Dissolving: the attraction of the particles of a solvent and a solute Soluble: can be dissolved Insoluble: cannot be dissolved Distillation: a process used to separate the solute and solvent in a solution Dilute: increasing the amount of solvent but the amount of solute stays the same Concentrated: increasing the amount of solute, but the amount of solvent stays the same Give an example of one solution that you can create at home. List the solute and the solvent in this solution At home, I could create a salt water solution. In this solution, the solute is the salt (because it is what is being dissolved) and the solvent is the water (because it is doing the dissolving) Why is water considered the “universal” solvent? Whay environmental implications can arise because of this? Water is considered the universal solvent because it can dissolve many materials. This can cause environmental problems because water can dissolve many harmful materials, due to pollution. Explain how distillation works using the particle theory. Distillation works because different substances have different boiling points. The particle theory states that when we heat up substances their particles move faster As particles move faster some of them break free into the air as vapour If this occurs at different temperatures for different substances, then we can boil one substance away and have the other left behind. This process is called distillation. Chapter 6 Saturated Solution: a solution that will not dissolved any more solute Unsaturated Solution: a solution where more solute will still dissolve Solubility: refers to how much solute will dissolve in a given amount of solvent. Supersaturated Solution: a solution where more solute than normally would dissolve has dissolved. This happens because of changing things like the temperature of the solvent. Rate of dissolving: how long it takes a solute to dissolve in a solvent It can be changed! How could you tell if a solution is saturated? Supersaturated? Unsaturated? Saturated: no more solute will dissolve in the solvent Unsaturated: more solute will dissolve in the solvent Supersaturated: the temperature had to be changed in order for more solute to dissolve than normal. Draw a diagram to show how a substance an dissolve in water. Name 3 ways that we can change the rate of dissolving. Give an example of each. Agitation: stirring the solution Increase the temperature of the solvent: boil the solvent before mixing in the solute Size of solute: Smash the solute into small pieces or a powder.