PowerPoint Version
advertisement

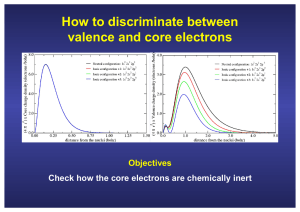
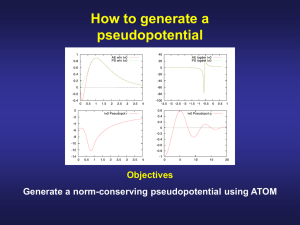
How to discriminate between valence and core electrons Objectives Check how the core electrons are chemically inert Description of the input file of the ATOM code for an all-electron calculation ae All-Electron calculation N 1s2 A title for the job core Chemical symbol of the nucleus 2s2 2p3 valence Number of core and valence orbitals Exchange-and correlation functional Principal quantum number Occupation Angular quantum number ca Ceperley-Alder (LDA) wi Wigner (LDA) hl Hedin-Lundqvist (LDA) bh von-Barth-Hedin (LDA) gl Gunnarson-Lundqvist (LDA) pb Perdew-Burke-Ernzerhof, PBE (GGA) rv revPBE (GGA) rp RPBE, Hammer, Hansen, Norvskov (GGA) ps PBEsol (GGA) wc Wu-Cohen (GGA) +s if spin (no relativistic) +r if relativistic bl BLYP Becke-Lee-Yang-Parr (GGA) am AM05 by Armiento and Mattson (GGA) vw van der Waals functional How to run an all-electron calculation with ATOM An explanation of the different files can be found in the ATOM User’s Guide (page 4) Run the code for different atomic configurations (neutral and ionic) Neutral configuration: 1s2 2s2 2p3 (N.0.ae.inp) Ionic configuration +1: 1s2 2s2 2p2 (N.+1.ae.inp) Ionic configuration +2: 1s2 2s2 2p1 (N.+2.ae.inp) Ionic configuration +3: 1s2 2s2 2p0 (N.+3.ae.inp) $../Utils/ae.sh N.0.ae.inp $../Utils/ae.sh N.1.ae.inp $../Utils/ae.sh N.2.ae.inp $../Utils/ae.sh N.3.ae.inp Plot the angularly integrated (multiplied by 4πr2) core and the charge densities $ gnuplot –persist charge.N-core.gplot $ gnuplot –persist charge.N-valence.gplot Core electrons are chemically inert All electron calculation for an isolated N atom Core charge density Valence charge density Core electrons are chemically inert All electron calculation for an isolated N atom Core charge density Valence charge density Core electrons are chemically inert All electron calculation for an isolated N atom Core charge density Valence charge density Core electrons are chemically inert All electron calculation for an isolated N atom Core charge density Valence charge density The core charge density remains unperturbed Although there are drastic modifications in the valence charge density Peak due to the 2s all-electron orbitals of N, (they have a node to be ortogonal with the 1s) To plot the all electron wave functions $ cd N.0.ae $ gnuplot –persist ae.gplot (To generate a figure on the screen using gnuplot) $ gnuplot ae.gps (To generate a postscript file with the figure) The radial parts that are plotted are the often called u’s in textbooks The s-wave functions also go to zero at the origin To identify the positions of the zero and the extrema $ vi OUT For each atomic orbital: • Position of the extrema (r extr) • Position of the zeros (r zero) • Position where the norm is 90 and 99% of the norm of the orbital Repeating the exercise for Si… Core electrons are chemically inert All electron calculation for an isolated Si atom Core charge density Core electrons are chemically inert All electron calculation for an isolated Si atom Core charge density Core electrons are chemically inert All electron calculation for an isolated Si atom Core charge density Core electrons are chemically inert All electron calculation for an isolated Si atom Core charge density