Electron Cloud notes
advertisement
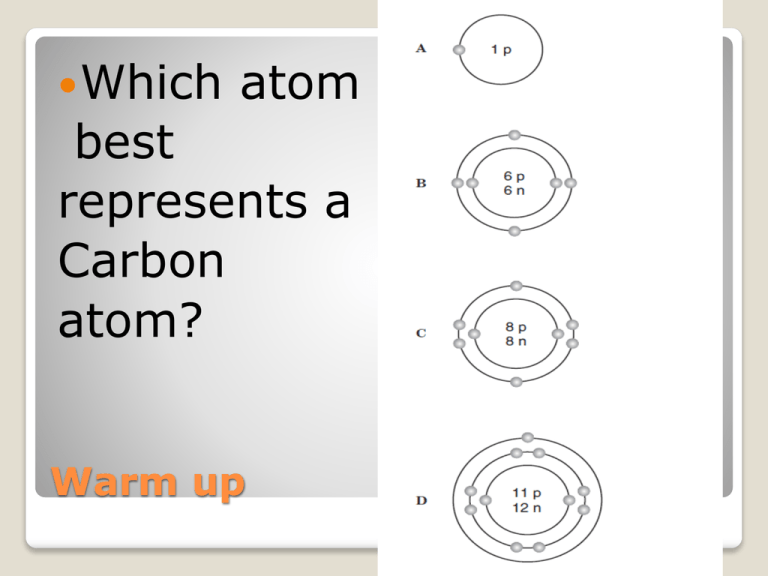
Which atom best represents a Carbon atom? Warm up Electron Cloud It’s a small world after all PLEASE!!!! Do not sing this terribly annoying song The electrons swirling around the nucleus of an atom are called the electron cloud. This ‘cloud’ contains all the electrons in a given atom. Valence electrons The electron cloud has different energy levels that electrons must follow in, called electron shells. We will only look at the first three. The following shells can hold: 1st shell-Up to 2 electrons 2nd shell- up to 8 electrons 3rd shell- up to 18 Electron cloud Vocab: Valence electrons: Electrons on the outermost shell of an atom. So how many valence electrons do these atoms electrons have? Valence model- a simple version of a more complicated system or concept. Models make something easier to understand. Models Just label the shells The Bohr modelshows ALL the electrons, protons, and neutrons in an atom. Draw in IAN The Bohr Model Scientist use a more simple way to represent atoms and their valence electrons than the Bohr model. An electron dot diagram shows ONLY the valence electrons of an atom and its name. Next, draw the same carbon atom using the electron dot diagram. Electron dot diagrams Draw in IAN the C (Carbon atom) and draw an arrow to the valence electrons. Bohr model Electron Dot model Bohr model vs. Electron dot Vocab: Rule of eight- all atoms want to have 8 valence electrons to fill up their outer shell. The atom will do whatever it can to make the rule of 8. This is a perfect time to introduce the atoms rule of eight The atom will give valence electrons away or steal from other atoms to make their outer shell have 8 electrons. Electrons, not money----> This is a perfect time to introduce the atoms rule of eight An atom reacts with another atom based on its # of valence electrons. (electrons on the outer shell) An atom is unstable and wants to react because of its # of valence electrons. EVERY chemical reaction happens because # of valence electrons This ‘stealing’ and ‘sharing’ is how ALL reactions happen!!! Lets do this on the board!!!!! Another example. The electron is stolen by the Cl atom and the Na atom wants to get rid of it Why? Remember those things called ions!











![Semiconductor Theory and LEDs []](http://s2.studylib.net/store/data/005344282_1-002e940341a06a118163153cc1e4e06f-300x300.png)