Matter and Energy Ch2 ANSWERS
advertisement

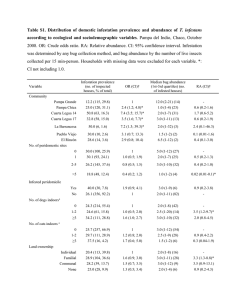
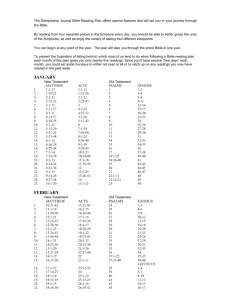
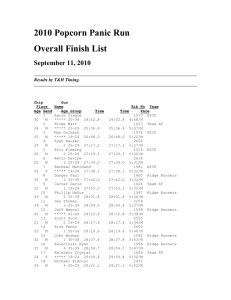
Matter and Energy Chapter 2 Section Reviews 2-1 (pg 47 #1-10) 2-2 (pg 55 #1-11) 2-3 (pg 64 #1-12) 2-1 (pg 47 #1-10) 1. What is the difference between potential energy and kinetic energy? Both are the capacity to do work. Kinetic energy is the energy an object has due to its motion. Potential energy is the energy of an object that has the potential to move because of its position. 2-1 (pg 47 #1-10) 2. What is the difference between heat and temperature? Heat is the total kinetic energy of the particles in a sample of matter. Temperature is a measure of the average kinetic energy of the particles in an object. 2-1 (pg 47 #1-10) 3. Explain what the equation E=mc2 signifies? The equation represents the amount of energy available when a quantity of matter is changed completely into energy. 2-1 (pg 47 #1-10) 4. What energy changes are considered chemical energy? The potential energy changes that result from the destruction and formation of chemical bonds are considered chemical energy. 2-1 (pg 47 #1-10) 5. 6. What is a system? A system is all of the components that are being studied at a given time. Define specific heat capacity. Specific heat capacity is the amount of heat required to raise 1 gram of the substance by 1 K. 2-1 (pg 47 #1-10) 7. What happens in terms of heat transfer when you hold a snowball in your hand? Heat passes from your hand, which is at a higher temperature, to the snowball, which is at a lower temperature. 2-1 (pg 47 #1-10) 8. a) Which of the metals listed requires the most energy to raise its temperature by 1°C? Aluminum. b) If energy is added at the same rate to containers all of the same mass made of copper, chromium, and lead, which container will show the greatest rise in temperature after 10 minutes? Lead 2-1 (pg 47 #1-10) 9. Convert the following Celsius temperatures to Kelvin temperatures? a) 100°C = 373K b) 785°C = 1058K c) 0°C = 273K d) -37°C = 236K 2-1 (pg 47 #1-10) 10. Convert the following Kelvin temperatures to Celsius temperatures? a) 273K = 0°C b) 1200K = 927°C c) 0K = -273°C d) 100K = -173°C 2-2 (pg 55 #1-11) 1. What activities are part of the scientific method? *Refer to the Scientific Method notes handout. 2-2 (pg 55 #1-11) 2. How does a hypothesis differ from a theory? A hypothesis is a testable explanation for observations. A theory is also an explanation of observations, but it is the result of repeated testing and revision of hypotheses. 2-2 (pg 55 #1-11) 3. State the law of conservation of mass? The total mass of the products of a reaction is the same as the total mass of the reactants. 2-2 (pg 55 #1-11) 4. What is a scientific law, and how does it differ from a theory? A scientific law reliably describes the observed behavior of the natural world but does not explain it. The theory explains the observed behavior. 2-2 (pg 55 #1-11) 5. Name two chemical compounds that were accidentally discovered, and describe what they are used for? Penicillin – treat infections. Saccharine – sugar substitute. 2-2 (pg 55 #1-11) 6. Why does a scientist include a control in the design of an experiment? A control is used to isolate the variable a scientist wants to study. 2-2 (pg 55 #1-11) 7. Give two reasons why scientists publish the results of their experiment. Research results are published so that researchers are credited for their work and so that other scientists can critique the results and attempt to replicate them. 2-2 (pg 55 #1-11) 8. How do models help chemists acquire knowledge about matter and energy? Models can be used to make predictions about the behavior of matter and energy. 2-2 (pg 55 #1-11) 9. What variables would a chemist need to control when setting up an experiment to determine whether or not a low temperature is required for TFE to form a polymer? Ex: the volume of the reaction vessel, the mass of the reactants, the pressure of the reaction system, and the composition of the reaction mixture. 2-2 (pg 55 #1-11) 9. What variables would a chemist need to control when setting up an experiment to determine whether or not a low temperature is required for TFE to form a polymer? Ex: the volume of the reaction vessel, the mass of the reactants, the pressure of the reaction system, and the composition of the reaction mixture. 2-2 (pg 55 #1-11) 10. Describe what is needed for a hypothesis to develop into a theory? Usually, many experiments followed by revision and refinement of the hypothesis are required. 2-2 (pg 55 #1-11) 11. Why is there no single scientific method? Scientists seldom follow a strict series of steps in their work. 2-3 (pg 64 #1-12) 1. How does accuracy differ from precision? Accuracy indicates how close a measurement is to the true value. Precision indicates how close repeated measurements are to each other. 2-3 (pg 64 #1-12) 2. What do the significant figures in a measurement indicate? The significant figures in a measurement are an indication of the precision of the measurement. 2-3 (pg 64 #1-12) 3. Explain the advantage of using scientific notation? Scientific notation makes it easier to write very large values and very small values. 2-3 (pg 64 #1-12) 4. What is an exact value? Give an example. Counts of items and conversion factors are exact values. Ex: 1000mm = 1m. 2-3 (pg 64 #1-12) 5. Describe a problem that may arise when you use a calculator to compute an answer. Calculators cannot make judgments about significant figures. Humans must do that. Therefore, calculator results must be rounded as needed. 2-3 (pg 64 #1-12) 6. Why is a burette rather than a graduated cylinder used when precise volumes are required in a lab procedure? Graduated cylinders can measure only to the nearest 0.1mL. Most burettes can measure to the nearest 0.01mL. 7. 2-3 (pg 64 #1-12) If you measure the mass of liquid as 11.50g and its volume as 9.03mL, how many significant figures should its density value have? The density value should have three significant figures because 9.03mL has only three significant figures. 2-3 (pg 64 #1-12) 8. How many significant figures are there in these expressions? a) 470km = 3 b) 0.0980m = 3 c) 30.8900g = 6 d) 0.09709kg = 4 2-3 (pg 64 #1-12) 9. Perform the following calculations, and express the answers in significant figures. a) 32.89g + 14.21g = 47.10g b) 34.09L – 1.230L = 32.86L c) 3.45 x 105g – 2.6 x 103g= 3.424 x 105g d) 1.8940cm x 0.0651cm = 0.123cm2 e) 24.897mi/0.8700h = 28.62mi/h 2-3 (pg 64 #1-12) 10. Express the following calculations in the proper number of significant figures. Use scientific notation where appropriate. a) 129g/29.2mL = 4.42g/mL b)(1.551mm)(3.260mm)(4.9001mm) = 24.78mm3 c) 35,000kJ/0.250s = 1.4 x 105kJ/s d) 0.367L + 2.5L + 1.6004L = 4.48L 11. 2-3 (pg 64 #1-12) A chemical process produces 653,550kJ of heat in 142.3 min. What is the rate of heat production in kJ/min? 4593kJ/min. 2-3 (pg 64 #1-12) 12. Express the following quantities in scientific notation. a) 277,088,000,000,000 atoms = 2.77088 x 1014atoms b) 0.000000000000839602g = 8.39602 x 10-13g c) 700,004mm = 7.00004 x 105mm