Sigmatropic Rearrangements
advertisement

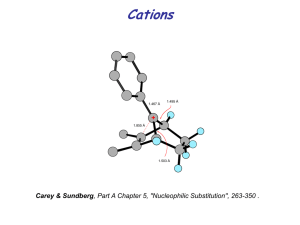
Chapter 10 Pericyclic Reactions(周环反应) Pericyclic Reactions • Continuous concerted reorganisation of electrons • 5 major categories: – – – – – Electrocyclic ring opening/closure Cycloaddition/cycloreversion reactions Cheletropic reactions (e.g. carbene addition) Group transfer reactions (e.g. H2 transfer) Sigmatropic rearrangements Sigmatropic Rearrangements • Migration of a s-bond across a conjugated p-system • [m,n] shift when the s-bond migrates across m atoms of one system and n of another 2 2 3 [1,3]-shift 1 3 1 1' R 1' R 2 2 1 R 1' 3 3' 2' 1 [3,3]-shift R 3 3' 1' 2' Conjugated π Systems Antibonding 4 3 p 3 2 nonbonding 2 p 1 1 2 p-orbitals 3 p-orbitals Bonding 4 p-orbitals Suprafacial/Antarafacial • Suprafacial migration: Group moves across same face R R' R' R R' R R R R' R R' R' • Antarafacial migration: Group moves from one face to the other R R' R' R R' R R' R R R' R R' FMO Analysis • [1,3] Sigmatropic Rearrangements: H migration + H H R R' 2 allyl anion HOMO R' R R' R 1s proton LUMO H H R R' Suprafacial migration FORBIDDEN R H R' Antarafacial migration ORBITALLY ALLOWED BUT H CANNOT BRIDGE DISTANCE FMO Analysis • [1,3] Sigmatropic Rearrangements: C migration + H3C CH3 R R' CH3 2p Carbon LUMO H H R' R R' R C H H H C H 2 allyl anion HOMO R R' R' R Suprafacial migration Retention at carbon Suprafacial migration Inversion at carbon FORBIDDEN ALLOWED FMO Analysis • [1,5] Sigmatropic Rearrangements X R X + X R' H R' R R' R 1s proton LUMO Suprafacial migration Antarafacial migration ALLOWED FORBIDDEN 3 pentadienyl anion HOMO C 2p carbon LUMO 3 pentadienyl anion HOMO Suprafacial migration Retention at Carbon ALLOWED C Antarafacial migration Inversion at Carbon ALLOWED Dewar-Zimmerman • Dewar-Zimmerman model: – Choose a set of 2p atomic orbitals and arbitrarily assign phase – Connect the orbitals in the starting material – Allow reaction to proceed according to postulated geometry and connect reacting lobes. – Count number of phase inversions: Odd = Möbius, Even = Hückel – Assign transition state as aromatic or antiaromatic based on number of electrons: System Aromatic Antiaromatic Hückel 4n + 2 4n Möbius 4n 4n + 2 – Aromatic = Thermally allowed (Photochemically forbidden) – Antiaromatic = Thermally forbidden (Photochemically allowed) Dewar-Zimmerman • [1,3]-H shift H H R' R Suprafacial: Two Phase Inversions Hückel Topology Four electrons FORBIDDEN H H • [1,5]-H shift H R H R' R Antarafacial: Three Phase Inversions Möbius Topology Four electrons ALLOWED H R' R H R' Suprafacial: Zero Phase Inversions Hückel Topology Six electrons THERMALLY ALLOWED Woodward-Hoffman • A ground-state pericyclic change is symmetry-allowed when the total number of (4q+2)s and (4r)a components is odd. • [1,5]-H shift – suprafacial H H s2s • [1,5]-H shift – antarafacial H H s2s H H p4s No. (4q+2)s = 1 No. (4r)a =0 Total =1 ALLOWED p4a No. (4q+2)s = 1 No. (4r)a =1 Total =2 FORBIDDEN Woodward-Hoffman • [1,7]-H shift – antarafacial H p6a H No. (4q+2)s = 1 No. (4r)a =0 Total =1 ALLOWED H s2s • [3,3] rearrangement R R Chair Boat s2 s R p2s p2s No. (4q+2)s = 3 No. (4r)a =0 Total =3 ALLOWED p2s s2 s R p2s No. (4q+2)s = 3 No. (4r)a =0 Total =3 ALLOWED [1,2] Sigmatropic Rearrangements • [1,2]-C shift to cation: Wagner-Meerwein Rearrangement R R 2p Carbon radical C 1 olefin radical cation + H OH Suprafacial migration: ALLOWED • [1,2]-C shift to anion: Wittig Rearrangement R R 2p Carbon radical 2 olefin radical anion R O R BuLi C O Li Suprafacial migration: FORBIDDEN Must be stepwise [2,3] Sigmatropic Rearrangements R R' * X R R' X Y Y R * X X, Y = C, N, O, S, Se, P • FMO Analysis 2 vinyl radical X Y Suprafacial migration ALLOWED 2 allyl radical Y R' [2,3] Sigmatropic Rearrangements • X=O, Y=C Wittig Rearrangement1 [2,3] BuLi O O Li Ph • + LiO Ph X=S, Y=C Sulfonium Ylide Rearrangement2 [2,3] BuLi S + S + Li S 1. 2. Ph Baldwin, JACS 1971, 93, 3556 Lythgoe, Chem. Comm. 1972, 757 S + S S [2,3] Sigmatropic Rearrangements • X=N, Y=C Ammonium Ylide Rearrangement3 (Stevens) R R BuLi + [2,3] + N N Li CN • R Me2 N + CN CN X=C, Y=C All-carbon Rearrangement4 [2,3] Cu(I) ROH -N2 O O N2 3. 4. O H Buchi, J. Am. Chem. Soc. 1974, 96, 7573 Smith, J. Org. Chem. 1977, 42, 3165 O OR [2,3] Sigmatropic Rearrangements • X=N, Y=O Meisenheimer Rearrangement5 R R [2,3] + Et N O Et • R Zn/HOAc Et N O OH Et X=S, Y=O Sulfoxide Rearrangement6 (MeO)3P MeOH Ph S + O [2,3] S Ph 5. 6. Tanabe, Tet. Lett. 1975, 3005 Evans, Accts. Chem. Res. 1974, 7, 147 O OH BuLi PhSCl [2,3] Sigmatropic Rearrangements • X=Se, Y=N Related Rearrangement7 Ph Ph Ph Se + Se N N Ts X=S, Y=N Related Rearrangement8 TsO TsO TsO NaN(Cl)Ts SPh 7. 8. NHTs Ph Ts • Ph MeOH [2,3] (MeO)3P MeOH Ph S Hopkins, Tet. Lett. 1984, 25, 15 Dolle, Tet. Lett. 1989, 30, 4723 [2,3] + N Ts N PhS N Ts Ts [2,3] Sigmatropic Rearrangements • Olefin Selectivity from starting olefin – 1,2-Disubstitution(E) R X Y R' R X R' H X H Y R' H H R R' X Y R and R’ prefer to sit in pseudo-equatorial positions9 2 LDA (E) selectivity: 75% O CO2 H 9. Y R Y X – R' R Nakai, Tet. Lett. 1981, 22, 69 HO CO2H [2,3] Sigmatropic Rearrangements • Olefin Selectivity from starting olefin – 1,2-Disubstitution(Z) R X Y R H H Y R' X Y R R' R' H Y R H X Y Generally, higher levels of 1,3 induction seen with Z olefins10 R Bu3Sn 10. X R' X – R' R BuLi O Still, J. Am. Chem. Soc. 1978, 100, 1927 R OH Only E isomer obtained [2,3] Sigmatropic Rearrangements • Olefin Selectivity from starting olefin – (E)-Trisubstituted R X Y R' R X R' H X H Y R Y X Y – R' R R' H H R R' X Y E transition state still generally preferred but R-Me interaction may cause significant destabilisation10 n-Bu Bu3Sn BuLi O >96% Z isomer n-Bu OH [2,3] Sigmatropic Rearrangements • Olefin Selectivity from starting olefin – (Z)-Trisubstituted R X Y R H H X R' X Y R' X Y – R' R Y R R' R' H H R X Y Again, generally higher levels of 1,3 induction seen with Z olefins due to highly destabilising R-R’ interaction [2,3] Sigmatropic Rearrangements • Olefin Selectivity from allylic position R X Y R' R X H Y X R' R' H R R > R' – R R' H Y X Y R' H R X Y May expect selectivity dependent on size difference of R vs. R’11 SLi BuLi S 11. R Rautenstrauch, Helv. Chim. Acta 1971, 54, 739 R (E):(Z) = 3:2 [2,3] Sigmatropic Rearrangements • Chiral Auxiliaries12 O O CH2OR O CH2OR Li BuLi O N ROCH2 – HO N ROCH2 O N ROCH2 96% de Via: O M O ROCH2 12. CH2OR N Katsuki, Tet. Lett. 1986, 27, 4577 CH2OR [2,3] Sigmatropic Rearrangements • Internal Relay of Stereochemistry13 O O O O O BuLi O O HO SnBu3 – HO ratio 79:6 Via: (Felkin-Ahn) C O H H O O 13. Bruckner, Angew. Chem. Int. Ed. 1988, 27, 278 [2,3] Sigmatropic Rearrangements • Steric Effects Y X t-Bu t-Bu – t-Bu Y X Y t-Bu Pseudo-equatorial attack generally favoured14 N2 S + Ph Cu(I) CO2 Et SPh t-Bu CO2 Et selectivity 91:9 14. Evans, J. Am. Chem. Soc. 1972, 94, 3672 X [2,3] Sigmatropic Rearrangements • Ring Expansion15 Cu(I) S • CO2 Et [2,3] S N2CHCO2Et + S CO2 Et Ring Contraction16 O Br Ph MeO O + N Ph MeOH O N Ph N " 15. 16. Vedejs, Accts. Chem. Res. 1984, 17, 358 Stevenson, Tet. Lett. 1990, 31, 4351 O " + N Ph O N Ph [3,3] Sigmatropic Rearrangements • FMO Analysis X X X Y Y Y 2 allyl radical Chair geometry ALLOWED • Dewar-Zimmerman Zero Phase Inversions Hückel Topology Six electrons THERMALLY ALLOWED Boat geometry ALLOWED [3,3] Sigmatropic Rearrangements X X X Y Y Y X, Y = C, O, N, etc X • Cope X O O Cope Rearrangement: Boat vs. Chair Transition State17 trans-trans 17. Claisen Doering, Roth, Tetrahedron 1962, 18, 67 trans-cis cis-cis [3,3] Sigmatropic Rearrangements • Cope Rearrangement: Boat vs. Chair Transition State H H Me Me H Me Me H Me Me Me Me H Me H H H Me Me H 10% trans-cis <1% trans-cis 99.7% H H Me Me H cis-cis H Me H Me Me H 90% Me H H trans-trans H Me H H Me Me trans-trans 0.3% [3,3] Sigmatropic Rearrangements • Cope Rearrangement: Use of ring strain18 H 5-20°C H – • Relief of ring strain upon rearrangement Oxy-Cope Rearrangement19 H keq ~ 105 220°C OH – 18. 19. OH Tautomerism shifts equilibrium to right Brown, Chem. Comm. 1973, 319 Marvell, Tet. Lett. 1970, 509 O [3,3] Sigmatropic Rearrangements • Oxy-Cope Rearrangement HO k1 O – – k2 HO O O 1010 < k2 < 1017 k1 Significant rate acceleration for anionic Oxy-Cope.20 Counter-ion also important OX OX 66°C THF H H MeO 20. Golob, J. Am. Chem. Soc. 1975, 97, 4765 OMe OX Half-life T/° C OH OLi ONa OK (66 yrs) No rxn 1.2 hrs 1.4 min 66 OK O- K+ 11 hrs 4.4 min 10 [3,3] Sigmatropic Rearrangements • X X Claisen Rearrangement O O X = C, H, O, N Thermodynamic driving force: (C-O) p-bond and (C-C) s-bond formation X=Heteroatom leads to higher exothermicity and reaction rate – – H OR ~30 O O ~20 H ~20 kcal/mol OR O ~30 kcal/mol O [3,3] Sigmatropic Rearrangements • Synthesis of allyl vinyl ethers21,22 OH Hg(OAc)2 O AcOHg OEt O OEt O Ph Ph O Cp2Ti 21. 22. Cl AlMe2 Watanabe, Conlon, J. Am. Chem. Soc. 1957, 79, 2828 Evans, Grubbs, J. Am. Chem. Soc. 1980, 102, 3272 O [3,3] Sigmatropic Rearrangements • Endocyclic Olefins23 Et O Via Chair intermediate: O 144°C Et O t-Bu t-Bu diastereoselection >87:13 t-Bu • Exocyclic Olefins24 O EtO O OEt O OEt t-Bu t-Bu – 23. 24. t-Bu ratio 52:48 Overlap equally good from either face Ireland, J. Org. Chem. 1983, 48, 1829 House, J. Org. Chem. 1975, 40, 86 [3,3] Sigmatropic Rearrangements • Olefin Selectivity CHO Me O O H CHO Me H R group prefers to sit in pseudo-equatorial position25 – CHO O 110°C R' CHO R' R' R 25. O R (E) Faulkner, J. Am. Chem. Soc. 1973, 95, 553 R (Z) R R’ (E):(Z) Me Et 90:10 Me i-Pr 93:7 Et Et 90:10 [3,3] Sigmatropic Rearrangements • Olefin Selectivity O Et Me X O X H Et X O Me Et O Et Me H X X Et O Me Me – Extra substituents lead to enhanced diastereoselection25 Larger X => better selectivity X (E):(Z) H 90:10 Me >99:1 MeO >99:1 Me2N >98:2 [3,3] Sigmatropic Rearrangements • Claisen Variants: Johnson Orthoester Claisen26 EtO OH MeC(OEt)3 OEt OEt O O OEt O + H • Claisen Variants: Eschenmoser Claisen27 MeO OH NEt2 MeO MeO O NEt2 NEt2 O Xylene 150°C 26. 27. Johnson, Faulkner, Peterson, J. Am. Chem. Soc. 1970, 92, 741 Eschenmoser, Helv. Chim. Acta 1964, 47, 2425 NEt2 O [3,3] Sigmatropic Rearrangements • Claisen Variants: Ireland Enolate Claisen28 O OTMS OH LDA O – TMSCl O O Substituted enolates afford an additional stereocentre29 R R' R' R R O R' O O H H OTBS H R R R' O OTBS Ireland, J. Am. Chem. Soc. 1976, 98, 2868 Ireland, J. Org. Chem. 1991, 56, 650 OTBS R' R O R' O H 28. 29. OTBS OTBS OTBS [3,3] Sigmatropic Rearrangements • Lewis Acid catalysed Claisen rearrangement LA O LA LA + O + O O LA Presence of Lewis Acid can influence rearrangement30 – R R O LA X R X R O O 30. O Yamamoto, J. Am. Chem. Soc. 1990, 112, 316 X Lewis Acid X R X LA O [3,3] Sigmatropic Rearrangements • Chiral Lewis Acid promoted Claisen rearrangement31 Ph (R)-1 1.1 - 2 eq Si(t-Bu)Ph2 Ph O 88% ee Al O DCM, -20°C O SiMe3 Me O SiMe3 Si(t-Bu)Ph2 (R)-1 • Enantioselective Claisen Rearrangements32 OBL2 O L2BBr i-Pr2NEt DCM 31. -20°C O >97% ee O Ph ArO2S O OBL2 L2BBr Et3N PhMe OH OH O Yamamoto, J. Am. Chem. Soc. 1990, 112, 7791 N N B SO2Ar Br -20°C O Ph L2BBr 96% ee 32. Corey, J. Am. Chem. Soc. 1991, 113, 4026 [m,n] Sigmatropic Rearrangements • [4,5] shift NMe2 Ph – • NMe2 Ph MeONa NMe2 Ph [4,5] [2,3] possible but [4,5] favoured. [2,5] and [3,4] forbidden [3,4] shift OMe MeO MeO O OH OH Key Retrons R • C=C + X: 1-6 Cope rearrangement R' X R R' R XH H X Retro-ene reaction R' • C=C + X: 1-5 R R R' Claisen rearrangement X X R' R • C=C + X: 1-4 X R [2,3] rearrangement Wittig X=O X H R' R' R' Ene reaction H X R'