Determining Chemical
advertisement
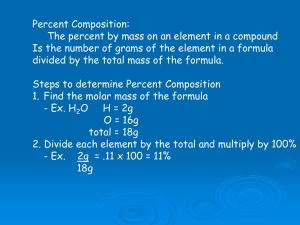
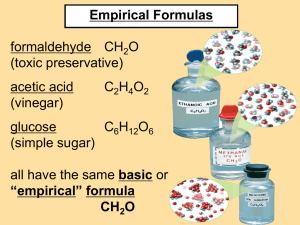
Determining Chemical Formulas Experimentally % composition, empirical and molecular formula Percentage Composition The mass of each element in a compound compared to the entire mass of the compound times 100 is the percent composition of that element. Example: What is the % composition of H and O in 1 mole of H2O? %H = 1.01 g H x 2 x 100 = 11% 18.02 g H2O =100% %O = 16.00 g O x 100 = 89% 18.02 g H2O Percentage Composition Example: A sample of an unknown compound with amass of 0.2370 g contains 0.0948 g of carbon, 0.126 g of oxygen, 0.016 g of hydrogen. What is the % composition of the compound? %C = 0.0948g X 100 = 40% 0.2370 g %O = 0.126 g x 100 = 53 % 0.2370 g %H = 0.016 g x 100 = 7% 0.2370 Empirical Formula The simplest whole number ratio of the elements in a compound is the empirical formula. Steps: 1. Find moles of each element in the compound. 2. Divide each mole value by the smallest mole value to find the mole ratio. 3. Write the formula using the mole ratios determined in step 2. A Poem Percent to grams Grams to moles Divide by smallest Multiply till whole Empirical Formula Example: A sample is analyzed and determined to contain 80 g C and 20 g H. What is the empirical formula? 1. 80 g C x 1 mole = 6.7 mol C 12.0 g 20 g H x 1 mole = 20 mol H 1.01 g Empirical Formula 2. 3. Mole ratios: C: 6.7 = 1 H: 20 = 2.98 ~ 3 6.7 6.7 CH3 is the empirical formula You may round the mole ratio if it is within 0.05 of a whole number. If it is not a whole number you must multiply all the mole ratios by a factor to get a whole number. Empirical Formula Example: What is the empirical formula of a compound that is 70% Fe and 30%O? 1. 70% Fe = 70 g Fe x 1 mole = 1.25 mol 55.85 g 30% O = 30 g O x 1 mole = 1.88 mol 16.00 g 2. Fe = 1.25/1.25 = 1 O = 1.88/ 1.25 = 1.5 Empirical Formula 3. Empirical formula must be in whole number ratios. Fe = 1 and O = 1.5, multiply both ratios by 2 Fe = 2 and O = 3, so the formula is: Fe2O3 Molecular Formula The formula of the actual molecular compound is the molecular formula. Steps: 1. Calculate the empirical mass. 2. Divide the molecular mass by the empirical mass. 3. Multiply each subscript in the empirical formula; by the number found in step 2. Molecular Formula Example: The molecular mass of a compound is found to be 180g/mol. If the empirical formula is CH2O, determine the molecular formula. 1. CH2O = 12.01g/mol + (1.01g/mol x 2) + 16.00 g/mol = 30.02 g/mol 2. I80g/mole =6 30.02 g/mole 3. CH2O x 6 = C6H12O6