Radioactivity
advertisement

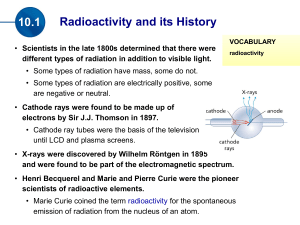
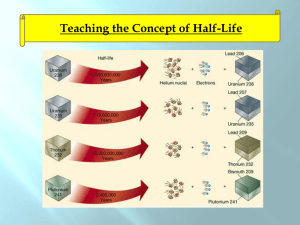
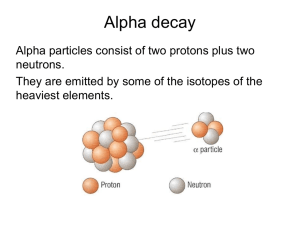
Do Now (3/17/14): 1. What are some words and images that come to mind when you hear the word “radioactivity”? 2. What is an isotope? 3. What makes an isotope different than its element? Radioactivity 4/23/12 Lesson Objectives Describe nuclear reactions and perform balancing of nuclear reactions by solving problems. Apply radioactivity equations by solving problems. Nuclear reaction A reaction in which the number of protons or neutrons in the nucleus of an atom changes. Atomic number Number of protons in the nucleus of the atom Mass number Sum of protons and neutrons in the nucleus of the atom Alpha decay Radioactive decay process in which the nucleus of an atom emits an alpha particle Alpha Particle Nucleus of a helium atom Beta decay Radioactive decay that occurs when a neutron is changed to a proton within the nucleus of an atom, and a beta particle and an antineutrino are emitted Gamma decay Radioactive process of decay that takes place when the nucleus of an atom emits a gamma ray. http://library.thinkquest.org/17940/texts/radioactivit y/radioactivity.html Isotope Atomic nuclei having the same number of protons but different number of neutrons All Elements Have Radioactive Isotopes All elements have more than one isotope Some isotopes of all elements are radioactive Some half-lives are so short that the isotope is not found naturally Radioactive Isotope display A Half-Life Is the Time Required for ½ the Atoms of a Substance to Undergo Radioactive Decay Applet Animation T1/2 = time for half the sample to disintegrate ---------------------------------------Assume T1/2 = 5 years ---------------------------------------Number of nuclei present at time t = 0: N0 = 1000 --------------------------------------When t = 5 yrs, N = 50 t = 10 yrs, N = 250 t = 20 yrs, N = 125. Calculate the half-life animation Applet Animation Half Life: Half-life: time needed for half of remaining mass of element to decay t (#halflives)T1 2 Example #1: Fermium-253 has a half-life of 0.334 seconds. A radioactive sample is considered to be completely decayed after 10 half-lives. How much time will elapse for this sample to be considered gone? Decay Rate T1/2=half life λ=decay rate 0.693 T1 2 Example #2: The half life of Zn-71 is 2.4 minute. If one had 100 g at the beginning, what is the decay rate of Zn-71? Mass remaining m m0e m=mass remaining Original mass t Example #3: The half life of Zn-71 is 2.4 minute. If one had 100 g at the beginning, how many grams would be left after 7.2 minutes elapsed? Practice: Use the rest of class to work on the paper: Radioactivity; problems: #2,5,6, and 7 Do Now (4/24/12): Pd-100 has a half-life of 3.6 days. If one had 6.02x1023 atoms at the start, how many atoms would be present Do Now (4/24/12): U-238 has a half-life of 4.46x109 years. How much U-238 should 10 be present in a sample 2.5 x10 years old, if 2 grams were present initially? Using Logarithms m m0e t m t e m0 Using Logarithms m t e m0 m ln t m0 Using Logarithms Solving for λ: m ln m0 t Using Logarithms Solving for t: m ln m0 t Decay series animation The Uranium Decay Series The only radium that exists today is that which is created as a result of the decay of uranium. Carbon-14 Production Neutron enters nucleus and kicks out a proton. 0n 1 + 7N14 ---------> 6C14 + 1p1 Carbon-14 Enters the Ecosystem Carbon Dating Since living organisms continually exchange carbon with the atmosphere in the form of carbon dioxide, the ratio of C-14 to C-12 approaches that of the atmosphere. From the known half-life of carbon-14 and the number of carbon atoms in a gram of carbon, you can calculate the number of radioactive decays to be about 15 decays per minute per gram of carbon in a living organism. Measuring the Age of Organic Matter A German tourist in the Italian Alps discovered the remains of the "Iceman" in the ice of a glacier in 1991. Calculating the Iceman's Age The current activity per gram of carbon half what it would be if the Iceman were alive. Since the half-life of carbon-14 is about 5700 years, the Iceman's remains are about 5700 years old. Radioactivity Equations N(t) = population at time t N(0) = population at time zero N0 = N(0) = decay constant N(t) = N0 e-t Example: N0 = 1000 = 2 x 10-3 years -1 When will N = 200? N = N0 e-t (1) e-t = N /N0 (2) ln (e-t) = ln (N /N0) (3) - t = ln (N /N0) (4) t = - [ln (N /N0)] / (5) = - [ln (200/1000)] /2 x10-3 (6) = 805 years Half-Life Problem The half-life of a radioactive substance is 10 hours. What is the decay constant, ? -------------------------------------------------------N = N0 e-t (1) 0.50 N0 = N0 e-10 e-10 = 0.50 (2) (3) ln(e-10) = ln(0.50) -10 = -0.693 = 0.0693 hrs-1 (4) (5) (6) Half-Life Problem From the previous problem, how much time will it take for the sample's activity to fall to only 20% of what it was originally? ---------------------------------------------N = 0.20 N0 (7) 0.20 N0 = N0 e-0.0693 t -0.0693 t = ln (0.20) t = 23 hours (8) (9) Decay Constant and Half-Life N = N0 e-t (1) 0.50 N0 = N0 e-T (T = half-life) e-T = 0.50 (3) ln(e-T) = ln(0.50) -T = -0.693 (4) (5) T = 0.693/ = 0.693/T (2) (6) (7) Half-Life Example 38Sr 90 (strontium-90) has a half-life of 28.5 years. How long will it take for 98% of a sample of strontium-90 to disappear? ----------------------------------------------------------------- = 0.693/T1/2 = 0.693 / 28.5 = 0.0243 years-1 0.02 = e-0.0243 t t = - ln(0.02) /0.0243 years-1 = 161 years Radioactivity Units A = number of disintegrations per second, activity A = N One becquerel (Bq) is one disintegration per second. One curie is the number of disintegrations per second (the "activity") of one gram of radium, or about 3.7 x 10 10 Bq. Units of Absorbed Radiation Rad: 10 milli-joules per kilogram 20 rads of X-rays doesn't do the same damage to humans as 20 rads of alpha particles. ---------------------------------------------Rem: an absolute biological damage unit Radiation Sickness Dose (rems) Effect 50-300 Sickness 400-500 Lethal 50% (LD50) Above 600 Lethal 100% (LD100) Calculate Rems from Rads (Relative Biological Effectiveness) Radiation a-particles R (rems/rad) 20 Neutrons 10 Protons 10 b-particles 1 g-rays 1 X-rays 1 Example: How many rads of protons will kill a person? -----------------------------600 rems is fatal RBE for protons is 10 Number of rads = 600 / 10 = 60 Example: One joule of energy per kilogram is absorbed in the form of neutrons. Will this prove fatal? -------------------------------1 rad is ten milli-joules 1 rad = 0.010 J Radon Poisoning Uranium in earth's crust decays to radium, which decays to radon. Radon is an odorless, tasteless, lighter-thanair gas which rises from the ground through cracks and fissures in the earth into homes. When breathed, the alphaemitting radon can cause cancer of the lung. Radon is the single greatest source of radiations for humans, providing about 200 milli-rems per year per person. Practice: Complete any four problems from the Radioactivity Worksheet When you are finished, raise your hand so I can stamp it Bring this paper to school with you this week!