******* 1
advertisement

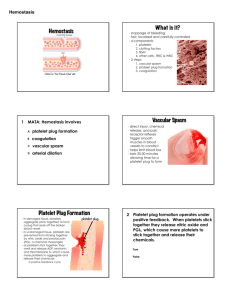
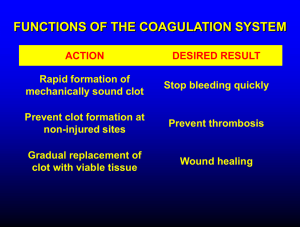
Hemostasis and Blood Coagulation Events in Hemostasis The term hemostasis means prevention of blood loss. Whenever a vessel is severed or ruptured, hemostasis is achieved by several mechanisms: (1) vascular constriction, (2) formation of a platelet plug, (3) formation of a blood clot as a result of blood coagulation, and (4) eventual growth of fibrous tissue into the blood clot to close the hole in the vessel permanently. Vascular Constriction Immediately after a blood vessel has been cut or ruptured, the trauma to the vessel wall itself causes the smooth muscle in the wall to contract; this instantaneously reduces the flow of blood from the ruptured vessel. The contraction results from (1) local myogenic spasm, (2) local autacoid factors from the traumatized tissues and blood platelets, and (3) nervous reflexes Mechanism of the Platelet Plug. When platelets come in contact with a damaged vascular surface, especially with collagen fibers in the vascular wall, the platelets themselves immediately change their own characteristics drastically. They begin to swell; they assume irregular forms with numerous irradiating pseudopods protruding from their surfaces; their contractile proteins contract forcefully and cause the release of granules that contain multiple active factors; they become sticky so that they adhere to collagen on the tissues and to a protein called von Willebrand factor that leaks into the traumatized tissue from the plasma; they secrete large quantities of ADP; and their enzymes form thromboxane A2. The ADP and thromboxane in turn act on nearby platelets to activate them as well, and the stickiness of these additional platelets causes them to adhere to the original activated platelets. Therefore, at the site of any opening in a blood vessel wall, the damaged vascular wall activates successively increasing numbers of platelets that themselves attract more and more additional platelets, thus forming a platelet plug. This is at first a loose plug, but it is usually successful in blocking blood loss if the vascular opening is small. Then, during the subsequent process of blood coagulation, fibrin threads form. These attach tightly to the platelets, thus constructing an unyielding plug Blood Coagulation in the Ruptured Vessel The third mechanism for hemostasis is formation of the blood clot. The clot begins to develop in 15 to 20 seconds if the trauma to the vascular wall has been severe, and in 1 to 2 minutes if the trauma has been minor. Activator substances from the traumatized vascular wall, from platelets, and from blood proteins adhering to the traumatized vascular wall initiate the clotting process. Fibrous Organization or Dissolution of the Blood Clot Once a blood clot has formed, it can follow one of two courses: (1) It can become invaded by fibroblasts, which subsequently form connective tissue all through he clot, or (2) it can dissolve. The usual course for a clot that forms in a small hole of a vessel wall is invasion by fibroblasts, beginning within a few hours after the clot is formed (which is promoted at least partially by growth factor secreted by platelets). This continues to complete organization of the clot into fibrous tissue within about 1 to 2 weeks. Mechanism of Blood Coagulation Basic Theory. More than 50 important substances that cause or affect blood coagulation have been found in the blood and in the tissues—some that promote coagulation, called procoagulants, and others that inhibit coagulation, called anticoagulants.Whether blood will coagulate depends on the balance between these two groups of substances. In the blood stream, the anticoagulants normally predominate, so that the blood does not coagulate while it is circulating in the blood vessels. But when a vessel is ruptured, procoagulants from the area of tissue damage become “activated” and override the anticoagulants, and then a clot does develop. General Mechanism .. General Mechanism three essential steps: (1) In response to rupture of the vessel or damage to the blood itself, a complex cascade of chemical reactions occurs in the blood involving more than a dozen blood coagulation factors. The net result is formation of a complex of activated substances collectively called prothrombin activator. (2) The prothrombin activator catalyzes conversion of prothrombin into thrombin. (3) The thrombin acts as an enzyme to convert fibrinogen into fibrin fibers that enmesh platelets, blood cells, and plasma to form the clot. Extrinsic Pathway for Initiating Clotting The extrinsic pathway for initiating the formation of prothrombin activator begins with a traumatized vascular wall or traumatized extravascular tissues that come in contact with the blood. This leads to the following steps, 1. Release of tissue factor. Traumatized tissue releases a complex of several factors called tissue factor or tissue thromboplastin. This factor is composed especially of phospholipids from the membranes of the tissue plus a lipoprotein complex that functions mainly as a proteolytic 2. Activation of Factor X—role of Factor VII and tissue factor. The lipoprotein complex of tissue factor further complexes with blood coagulation Factor VII and, in the presence of calcium ions, acts enzymatically on Factor X to form activated Factor X (Xa). 3. Effect of activated Factor X (Xa) to form prothrombin activator—role of Factor V. The activated Factor X combines immediately with tissue phospholipids that are part of tissue factor or with additional phospholipids released from platelets as well as with Factor V to form the complex called prothrombin activator.Within a few seconds, in the presence of calcium ions (Ca++), this splits prothrombin to form thrombin, and the clotting process proceeds as already explained. At first, the Factor V in the prothrombin activator complex is inactive, but once clotting begins and thrombin begins to form, the proteolytic action of thrombin activates Factor v. This then becomes an additional strong enzyme. accelerator of prothrombin activation. Thus, in the final prothrombin activator complex, activated Factor X is the actual protease that causes splitting of prothrombin to form thrombin; activated Factor V greatly accelerates this protease activity, and platelet phospholipids act as a vehicle that further accelerates the process. Note especially the positive feedback effect of thrombin, acting through Factor V, to accelerate the entire process once it begins. Intrinsic Pathway for Initiating Clotting The second mechanism for initiating formation of prothrombin activator, and therefore for initiating clotting, begins with trauma to the blood itself or exposure of the blood to collagen from a traumatized blood vessel wall. Then the process continues through the series of cascading reactions. 1. Blood trauma causes (1) activation of Factor XII and (2) release of platelet phospholipids. Trauma wall collagen alters two important clotting factors in the blood: Factor XII and the platelets.When Factor XII is disturbed, such as by coming into contact with collagen or with a wettable surface such as glass, it takes on a new molecular configuration that converts it into a proteolytic enzyme called “activated Factor XII.” Simultaneously, the blood trauma also damages the platelets because of adherence to either collagen or a wettable surface (or by damage in other ways), and this releases platelet phospholipids that contain the lipoprotein called platelet factor 3, which also plays a role in ubsequent clotting reactions.to the blood or exposure of the blood to vascular Intrinsic Pathway for Initiating Clotting wall collagen alters two important clotting factors in the blood: Factor XII and the platelets.When Factor XII is disturbed, such as by coming into contact with collagen or with a wettable surface such as glass, it takes on a new molecular configuration that converts it into a proteolytic enzyme called “activated Factor XII.” Simultaneously, the blood trauma also damages the platelets because of adherence to either collagen or a wettable surface (or by damage in other ways), and this releases platelet phospholipids that contain the lipoprotein called platelet factor 3, which also plays a role in subsequent clotting reactions. 2. Activation of Factor XI. The activated Factor XII acts enzymatically on Factor XI to activate this factor as well, which is the second step in the intrinsic pathway. This reaction also requires HMW (high-molecularweight) kininogen and is accelerated by prekallikrein. 3. Activation of Factor IX by activated Factor XI. The activated Factor XI then acts enzymatically on Factor IX to activate this factor also. 4. Activation of Factor X—role of Factor VIII. The activated Factor IX, acting in concert with activated Factor VIII and with the platelet phospholipids and factor 3 from the traumatized platelets, activates Factor X. It is clear that when either Factor VIII or platelets are in short supply, this step is deficient. Factor VIII is the factor that is missing in a person who has classic hemophilia, for which reason it is called antihemophilic factor. Platelets are the clotting factor that is lacking in the bleeding disease called thrombocytopenia. 5. Action of activated Factor X to form prothrombin activator—role of Factor V. This step in the intrinsic pathway is the same as the last step in the extrinsic pathway. That is, activated Factor X combines with Factor V and platelet or tissue phospholipids to form the complex called prothrombin activator. The prothrombin activator in turn initiates within seconds the cleavage of prothrombin to form thrombin, thereby setting into motion the final clotting process, as described earlier.