Empirical and Molecular Formulas
advertisement

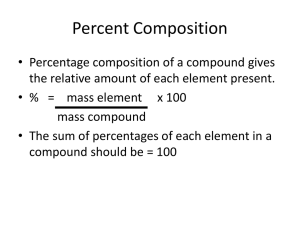
Review Chemical formulas are very important; they: state which elements are in the molecule give the exact number of atoms of each element that are in the molecule may give an indication as to how the elements are bonded together The law of definite proportions When elements combine to form compounds, they do so in a definite proportion. it is also sometimes called the law of constant composition. The percentage composition of an element can be expressed as: Examples Water has the chemical formula H2O. In terms of mass, its molecule is always made up of 11% hydrogen and thus the remaining mass (89%) must be oxygen. Notice how much more massive the oxygen atom is in this image of the water molecule. Similarly, CO2 is always made up of 27.3% carbon. Percentage composition of compounds The percentage composition is the percentage of an element in a compound, in relation to its total mass. Example 1 Calculate the percentage composition (by mass) of Al2O3. MM(Al2O3) = 2(27 g/mol) + 3(16 g/mol) = 102 g/mol % Al = 2(27 g/mol)/102 g/mol x 100% = 52.9% % O = 3(16 g/mol)/102 g/mol = 47.1% So Al2O3 is 52.9% aluminum and 47.1% oxygen. Example 2 Calculate the percentage composition by mass of sodium sulphate, Na2SO4. MM(Na2SO4) = 2(23 g/mol) + 1(32.1 g/mol) +4(16 g/mol) = 142.1 g/mol % Na = 2(23 g/mol)/142.1 g/mol = 32.4% % S = 1(32.1 g/mol)/142.1 g/mol = 22.6% % O = 4(16 g/mol)/142.1 g/mol = 45.0% So, sodium sulphate is 32.4% sodium, 22.6% sulphur and 45.0% oxygen. Check Your Understanding 1. 13.0 g of tin is burned in the presence of oxygen to form 16.5 g of tin (IV) oxide. Which of the following represents the percentage composition of tin by mass in this compound? a) 33.5% b) 44.1% c) 78.8% d) 85.1% 2. 5.3 g of mercury (II) oxide is decomposed to form 4.9 g of mercury. What is the percentage composition (mass) of mercury in the compound? a) 7.5% b) 16.0% c) 59.6% d) 92.5% Empirical Formula A chemical's empirical formula indicates the simplest ratio in which the different atoms are combined. It is also called the simplest formula. For example the empirical formula for acetylene (C2H2) is CH, since there is 1 C for every H. Empirical Formula (con’t) However, benzene also has the empirical formula CH; but its chemical formula is C6H6. Benzene and acetylene are not the same chemical; each compound is unique in terms of physical and chemical properties. Steps for finding the empirical formula: 1. Consider the amounts you are given as being in units of grams. 2. If percentages are given instead of grams, assume them to be grams (since percentages add up to 100, then your sample's mass will be 100 g). 3. Convert the grams to moles for each element. 4. Find the smallest whole number ratio of moles for each element. Example 3 A sample of calcium chloride contains 1.82 g of calcium and 3.23 g of chlorine. What is the empirical formula for the compound? Ca: 1.82 g/40.1 g/mol = 0.0454 mol Cl: 3.23 g/35.5 g/mol = 0.0910 mol mole ratio = Cl:Ca = 0.0910 mol:0.0454 mol = 2:1 So there are 2 mol of Cl for every 1 mol of Ca The empirical formula is CaCl2. Example 4 The elemental analysis of an unknown organic compound returns the following data: %C = 64.6% %H = 10.8% %O = 24.6% What is the empirical formula for this compound? Assuming a 100 g sample, divide the percentages by the molar masses of the elements to determine the number of moles of each element present in the formula: C: 64.6 g / 12.001 g/mol = 5.38 mol C H: 10.8 g / 1.008 g/mol = 10.7 mol H O: 24.6 g / 16.00 g/mol = 1.54 mol O Divide the number of moles by the smallest number of moles to determine the relative ratios of the elements. C:H:O = 5.38 / 1.54 : 10.7 / 1.54 : 1.54 / 1.54 = 3.49:6.95:1.00 This gives the formula C3.49H6.95O1.00 Example 4 (con’t) Remember that elements only combine in whole number ratios. If the ratios are close to whole numbers then we know the empirical formula. If they are not, then you must multiply the ratios by a whole number until all are relatively close to an integer value. Here, we multiply the above formula by 2. Therefore, the empirical formula is C6.98H13.9O2.00 You can round this off to C7H14O2 Check Your Understanding 3. Suppose a compound is analyzed and reveals that is composed of 85.7% carbon and 14.3% hydrogen. Find the empirical formula for this compound. 4. Determine the empirical formula for a compound which is broken down into 6.16 g of sulphur and 8.84 g sodium. Molecular Formula The molecular formula indicates the number of atoms of each element in a molecule. It is determined using the empirical formula and the molar mass. Steps for finding the molecular formula: 1. Determine the compound's empirical formula. 2. Divide the compound's molar mass by the mass of the empirical formula. 3. Multiply each subscript in the empirical formula by this number. Example 5 Hydrogen peroxide is made of 5.03% hydrogen and 79.87% oxygen. Its molar mass is 34.01 g/mol. Find its molecular formula. First, you need to find the empirical formula: Percentages are given, so you can assume that you are working with a 100 g sample that is 5.03 g of hydrogen and 79.87 g of oxygen. H: 5.03 g/1.0079 g/mol = 4.99 mol O: 79.87 g/15.9994 g/mol = 4.99 mol mole ratio = H:O = 4.99 mol:4.99 mol = 1:1 So there is 1 mol of H for each mole of O. The empirical formula is HO. Now, you can find the molecular formula: MM(HO) = 17.0073 g / mol MM(hydrogen peroxide) = 34.01 g/mol MM(hydrogen peroxide)/MM(HO)=34.01 g/mol /17.0073 g/mol =1.9997 =2 Therefore, each subscript in the empirical formula is multiplied by 2. That gives H2O2. So, the molecular formula of hydrogen peroxide is H2O2. Check Your Understanding 5. If 4.04 g of nitrogen combine with 11.46 g of oxygen to produce a compound with a molar mass of 108.0 g/mol, what is the molecular formula of this compound? Check Your Understanding 6. Determine the molecular formula of a compound with an empirical formula of NH2 and a molar mass of 32.06 g/mol. Here is a comparison between three molecular formulas that have the same empirical formula of CH2O. Notice how they have vastly different properties. Substance Formaldehyde Acetic acid Glucose Empirical formula CH2O CH2O CH2O Molecular formula CH2O C2H4O2 C6H12O6 biological preservative vinegar sweetener State gas liquid solid Molar mass (g/mol) 30 60 180 Melting point (°C) -92 16 140 Boiling point (°C) -19.5 118 decomposes Use Physical properties