Phase IB Trial of Carboxyamidotriazole Orotate (CTO) and Temozolomide for Recurrent Malignant Glioma (MG):
A Novel Mechanism for Modulation of Multiple Oncogenic pathways
Antonio M. P. Omuro1, Thomas J. Kaley1, Elena Pentsova1, Lisa M. DeAngelis1, Walter J. Urba2, Matthew H. Taylor3, Barry D. Anderson4, Greg Gorman5, Sean McLean4, Rashida A. Karmali6
1Memorial
Sloan-Kettering Cancer Center, New York, NY; 2Earle A Chives Research Institute and Providence Cancer Center, Portland, OR; 3Oregeon Health & Science University, Portland, OR; 4Theradex, Princeton, NJ; 5Samford University, Birmingham, AL; 6Tactical Therapeutics,New York, NY
INTRODUCTION
Multiple signal transduction pathways are
activated in malignant glioma (MG) and
glioblastoma (GBM). CTO is an oral inhibitor of
non-voltage-dependent calcium signaling that
modulates several receptor-mediated calcium –
dependent signaling pathways, including EGFR,
MEK, RAS, HDAC, HSP90, WNT/B-catenin, Akt,
ERK, VEGF and Bcr-Abl. (Kohn, 1997; Berlin,
1997; Corrado 2012; Karmali, 2013).
This proof-of-concept Phase IB trial follows
completion of the CTO Phase I safety study at
doses of 75-427mg/m2/day in solid tumors, in
which a safe toxicity profile, predictable
pharmacokinetics and responses in various
refractory tumors with different mutations were
observed. (ASCO 2013).
A possible mechanism of resistance to targeted
and chemo-therapeutics is the existence of
redundant signaling pathways.
CTO crosses the blood-brain barrier, in
preclinical studies CTO alone has shown activity
in melanoma and GBM xenografts, and robust
synergism with temozolomide (TMZ) prompting
this proof-of-concept Phase IB (Figure 1).
(Karmali, 2011, Karmali 2013).
STUDY DESIGN
Study Objectives
- Determine the safety and MTD of CTO and TMZ
- Determine the Tumor Response of MG and
GBM according to the MacDonald Criteria
- Determine the pharmacokinetics of CTO and
TMZ when co-administered
- Confirm the proof-of –concept with evidence
of synergism between CTO and TMZ
- Investigate effect of CTO and TMZ on growth of
tumors with various genotypes (MGMT +/-)
- Investigate the effect of CTO and TMZ on gene
expression in anagen hair from treated patients
Study Design
- Combination of escalating dose of CTO with
fixed dose of TMZ (150mg/m2)
- “3+3” design, CTO administered orally at a
starting dose of 219mg/m2/day
Table 1: Summary of Cohorts
Characteristics
Key Inclusion Criteria
- Histologically proven, recurrent MG
- Measurable tumor on a gadoliniumenhanced MRI
- ECOG 0, 1, 2
- Life expectancy > 8 Weeks
- No CYP3A4 inhibitors or inducers
Study Treatment
- CTO administered daily, 28-day cycle
- TMZ administered days 1-5 of each cycle
Evaluations
- Response: Gadolinium-enhanced MRI
every two cycles
- Safety: Adverse events, vital signs, ECG,
clinical laboratory tests, physical exams
- Pharmacokinetics, pharmacogenomics
Table 2: Patient Demographics
N(%)
Study Enrollment
Cohort 1 (219mg/m2/day), n= 4
Age (Years)
Median
51
Cohort 2 (285mg/m2/day), n= 3
Range
27-77
Cohort 3 (370mg/m2/day), n= 3
RESULTS
Table 4: Summary of Adverse Events
Table 3: TEAEs in >10% of Patients
Treatment-Emergent
Adverse Events
N(%)
Safety evaluable patients
No. of patients with TEAEs
14 (100%)
14 (100%)
Constipation
Fatigue
Nausea
Vomiting NOS
Headache NOS
Headache NOS
Dizziness
Dry skin
Dysgeusia
Convulsions NOS
Diarrhoea NOS
Myalgia
Hypophosphataemia
Anxiety
Micturition urgency
7 ( 50.0%)
7 ( 50.0%)
6 ( 42.9%)
5 ( 35.7%)
4 ( 28.6%)
4 ( 28.6%)
3 ( 21.4%)
3 ( 21.4%)
2 ( 14.3%)
2 ( 14.3%)
2 ( 14.3%)
2 ( 14.3%)
2 ( 14.3%)
2 ( 14.3%)
2 ( 14.3%)
Summary of
Adverse Events
N(%)
Safety evaluable patients
Patients
14 (100%)
14 (100%)
With Any Adverse Event
With Possibly, Probably, Or
Definitely CTO Related
Adverse Events
With Possibly, Probably, Or
Definitely TMZ Related
Adverse Events
With Severity Grade 3, 4 or
14 (100%)
10 ( 71.4%)
With Severity Grade 3, 4 or
5 for CTO Related Adverse
Events
With Any Serious Adverse
Events
Who Died Due to Adverse
Events
1 ( 7.1%)
13 ( 92.9%)
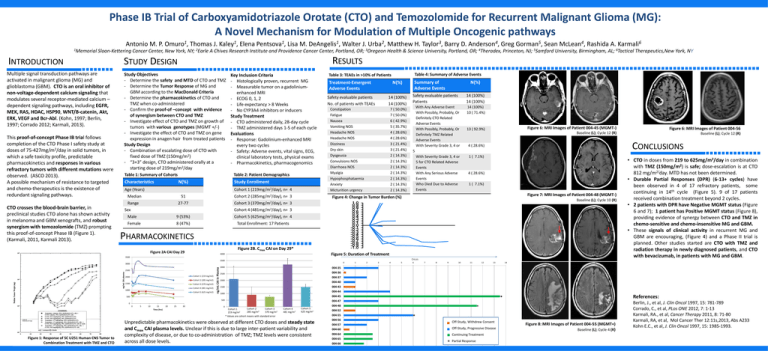
Figure 6: MRI Images of Patient 004-45 (MGMT-)
Figure 6: MRI Images of Patient 004-56
Baseline (L); Cycle 12 (R)
Baseline (L); Cycle 12 (R)
CONCLUSIONS
4 ( 28.6%)
5
4 ( 28.6%)
1 ( 7.1%)
Figure 7: MRI Images of Patient 004-48 (MGMT-)
Figure 4: Change in Tumor Burden (%)
Baseline (L); Cycle 10 (R)
Cohort 4 (481mg/m2/day), n= 3
Sex
Male
9 (53%)
Cohort 5 (625mg/m2/day), n= 4
Female
8 (47%)
Total Enrollment: 17 Patients
PHARMACOKINETICS
Figure 2B. Cmax CAI on Day 29*
Figure 2A CAI Day 29
Figure 5: Duration of Treatment
4000
3500
CYCLES
3500
0
ng/mL CAI plasma
2500
Cohort 1 (219 mg/m2)
2000
Cohort 2 (285 mg/m2)
1500
Cohort 3 (370 mg/m2)
Cohort 4 (481 mg/m2)
1000
Cohort 5 (625 mg/m2)
ng/mL CAI in Plasma
3000
3000
1
2
3
4
5
6
7
8
9
10
11
12
13
10
15
Time (hrs)
20
25
30
14
004-37
2000
004-40
004-43
1500
004-44
1000
500
004-47
0
004-48
0
5
• CTO in doses from 219 to 625mg/m2/day in combination
with TMZ (150mg/m2) is safe; dose-escalation is at CTO
812 mg/m2/day. MTD has not been determined.
• Durable Partial Responses (DPR) (6-13+ cycles) have
been observed in 4 of 17 refractory patients, some
continuing in 14th cycle (Figure 5). 9 of 17 patients
received combination treatment beyond 2 cycles.
• 2 patients with DPR have Negative MGMT status (Figure
6 and 7); 1 patient has Positive MGMT status (Figure 8),
providing evidence of synergy between CTO and TMZ in
chemo-sensitive and chemo-insensitive MG and GBM.
• These signals of clinical activity in recurrent MG and
GBM are encouraging, (Figure 4) and a Phase II trial is
planned. Other studies started are CTO with TMZ and
radiation therapy in newly diagnosed patients, and CTO
with bevacizumab, in patients with MG and GBM.
004-36
2500
004-45
0
004-35
500
Figure 1: Response of SC U251 Human CNS Tumor to
Combination Treatment with TMZ and CTO
Cohort 2
Cohort 3
Cohort 1
2
2
285
mg/m
370 mg/m2
219 mg/m
* Values are cohort means with standard error
Cohort 4
481 mg/m2
Cohort 5
625 mg/m2
Unpredictable pharmacokinetics were observed at different CTO doses and steady state
and Cmax CAI plasma levels. Unclear if this is due to large inter-patient variability and
complexity of disease, or due to co-administration of TMZ; TMZ levels were consistent
across all dose levels.
*
004-52
004-55
004-56
004-57
*
Off-Study, Withdrew Consent
004-60
Off-Study, Progressive Disease
004-64
Continuing Treatment
004-65
004-66
References:
*
Partial Response
Figure 8: MRI Images of Patient 004-55 (MGMT+)
Baseline (L); Cycle 4 (R)
Berlin, J., et al, J. Clin Oncol 1997, 15: 781-789
Corrado, C., et al, PLos ONE 2012, 7: 1-13
Karmali, RA., et al, Cancer Therapy 2011, 8: 71-80
Karmali, RA, et al, Mol Cancer Ther 12:11s,2013, Abs A233
Kohn E.C., et al, J. Clin Oncol 1997, 15: 1985-1993.