Venipuncture PP
advertisement

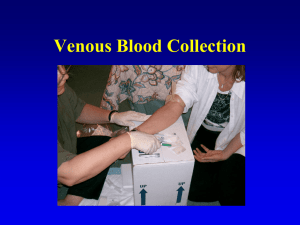
VENIPUNCTURE Chapter 20, pages 585 – 590, in CTVT OBJECTIVES Describe patient preparation, positioning, and procedures for blood collection from peripheral veins Describe indications and procedures for collection of arterial blood samples in small animals Become familiar with blood collection techniques and protocols General Principles for Collection Of Samples for Laboratory Testing Blood and urine samples should be obtained before treatment Supplies are appropriate for the sample and gathered before collection is started Site of collection is free of disease and debris Area is prepared properly 3 Blood Sample Collection General guidelines Minimum stress to patient is required Avoid hemolyzed sample Fill vacuum tube with syringe Anticoagulant tubes mixed gently Serum tubes set at an angle and allowed to clot 4 Patient Condition Examples Is the animal in Shock? Dehydration status Is the animal Breathing? Is the animal Normal and Healthy? Are all the veins blown? Is the animal a neonate? Temperament Examples Is the animal trying to take your head off? Is the animal calm and laid back? Is the animal conscious? Is the animal dying? Remember “flight or fight” syndrome? Venipuncture Supplies Needles (Get more than one) Syringes (Get more than one) Cotton balls (or gauze) in alcohol Vet Wrap, gauze or dry cotton balls (Band-aid) Muzzle or muzzles (E-collars can also act as great restraint devices) Proficient person to restrain the animal Hydrogen Peroxide (optional and if available) Venous Blood Sample Only through experience does one learn to collect a blood sample quickly with minimal trauma to the vessel and minimal stress and discomfort to the patient Proper RESTRAINT is as important as the venipuncture technique You must always insert the needle bevel facing up, into the vein Unlike injections – you ‘hold off’ the ENTIRE time, until you obtain all the blood you need Venous Blood Sample Performed with a needle and syringe or a vacutainer collection system The method and needle gauge depend on: Vessel size Amount of blood required Intended use of the sample technician preference You should use the largest needle possible for the vein chosen and direct the bevel upward Blood Collection The reason you are drawing blood will dictate: Limb to be used Amount of blood you will need Time frame involved Your expertise Always think about the situation before you draw blood Simple Guidelines Smaller gauge needles are used with smaller or more fragile vessels, or multiple venipunctures The amount of negative pressure applied to aspirate the blood into the syringe must not be excessive Forceful retraction of the plunger may result in hemolysis of the red blood cells as they pass through the needle. Remember these little guys are fragile. Not only can you collapse the vein but your lab results may be effected Guidelines Before venipuncture, the hair and skin over the vessel are wiped with a cotton ball saturated with 70% isopropyl alcohol (or you can shave the hair) This helps to remove dirt, causes vasodilation, and improves visualization of the vein In animals with dense hair coats, the vessel may be easier to identify if the hair over the vessel if parted with an alcohol ball or shaved Peripheral Vein Blood Collection Introduce the needle into the occluded vessel as far distally as possible. Use a quick but smooth “poke” Do not “jab” into the vein – you will go through it If your first attempt is unsuccessful reinsert the needle more proximal to the previous entry site. In other words, make your first attempt to draw blood as far down the leg as possible. If you miss or damage the vein you can always make other attempts moving upward along leg Cephalic Venipuncture The cephalic vein is located on the cranial aspect of the foreleg The animal is restrained in sternal recumbency or in a standing position.Your larger breed dogs prefer to remain standing or be seated with the foreleg in extension If you are utilizing the right cephalic vein the restrainer is positioned on the animal’s left side Use your thumb to help stabilize the vein so it does not roll Lateral Saphenous Vein Medial Saphenous Venipuncture in the Cat This vein is located on the medial aspect of the rear leg, and is used to obtain small volumes of blood, primarily in felines If the right vein is used place the cat in right lateral recumbency with the left rear leg abducted The vein is occluded with pressure applied by the restrainer’s left hand in the right marginal region (kitty karate chop) Wipe the leg with alcohol and part the fur until you see the vein Blood is collected with a 22- to 25- gauge needle attached to a 1- or 3-ml syringe Medial Saphenous Venipuncture in the Cat Firm pressure is applied to the puncture site for at least 60 seconds after venipuncture. It is extremely important to do this because the medial saphenous veins are especially prone to hematoma formation Even the best phlebotomist can cause a hematoma on a cat Medial Saphenous Vein Jugular Venipuncture Collection Patient's head is raised exposing the neck Your initial attempt should be made in the caudal third of the jugular vein Subsequent attempts can be made in a more cranial region If the vessel is damaged in the distal portion of the vein, a more proximal region is still patent and usable for blood collection The jugular furrow can be palpated just lateral to the trachea Person drawing the blood must “Hold Off ” • Palpation of the jugular vein in lieu of visualization may be necessary in some animals • Wiping the neck with alcohol helps to visualize the vein. In cats, water is sometimes used because the smell of alcohol is often repugnant to them. The smell sometimes makes them gag After the Blood Collection; With a sense of urgency! Detach needle from your syringe Remove stopper from collection tube Transfer blood into the tube If utilizing a anticoagulant tube, gently invert the tube a few times to mix the contents. Do NOT SHAKE it! Your tube must be filled at least half way to achieve the appropriate blood/anticoagulant ratio Always dispose of your needles/syringes in the “sharps” container Marginal Ear Vein – At Home Glucose testing Arterial Blood Sampling WHY? •Best way to assess pulmonary function •Blood Gasses tell us about the patient’s ability to ventilate and oxygenate WHAT? •Measure CO2 (ventilation) and O2 (oxygenation) •Performed on a Blood Gas analyzer Common errors with blood collection and handling Rapid or forceful aspiration of the blood, especially through a needle less than 22g Traumatic aspiration of blood Spraying the blood through the needle into a second vial. The needle and tube stopper should be removed and the blood runs gently down the inside of the vial Water present in the syringe, needle, or tube, causing osmotic damage to the cells Collecting too little or to much blood for the amount of anticoagulant present, resulting in dilution error or direct cellular damage from concentration anticoagulant Slow collection or delayed transfer to anticoagulant, allowing clumping of platelets and clot formation Improper or incomplete mixing of anticoagulant. The tube should be gently but thoroughly rotated by hand or on an automatic tube rotator or it may be rolled across a flat surface Excessive physical force, such as shaking, jarring, or dropping VIDEOS ON VENIPUNCTURE http://www.youtube.com/watch?v=AND813sHUks http://www.youtube.com/watch?v=ofw6pnP04zY&feature =related http://www.youtube.com/watch?v=iUnabo1KmgI