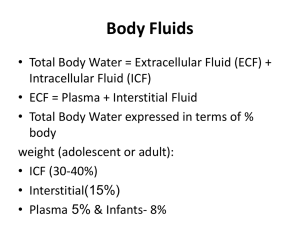
Introduction to the Principles of Fluid and Electrolyte Therapy
advertisement
Fluid and Electrolyte Therapy in the Pediatric Patient Steve Piecuch MD, MPH Department of Pediatrics Lincoln Medical Center Maintenance Requirements Introduction to the Principles of Fluid and Electrolyte Therapy Important to understand the underlying physiologic principles of a therapy commonly employed in pediatrics Understanding basic principles essential for the understanding of the management of more complex disorders such as: – Cholera – Dengue – Pyloric stenosis – DKA – Hyperosmotic non-ketotic coma Crystalloid and Colloid Crystalloid: Water and electrolyte solution » Does not remain within the intravascular space but rather distributes to the entire extracellular space » Only impacts on the intracellular space if it causes a change in extracellular osmolarity – E.g.: 0.9% NaCl, D5 0.3% NaCl Colloid: Contains large particles which tend to remain within the blood vessels » Colloid preferentially expands the intravascular space because the particles exert oncotic force which retains water within the intravascular space – E.g.: 5% albumin, blood, dextran solution Isotonic Saline Solution Isotonic saline solution: Solution such as 0.9% NaCl or Ringer’s lactate with a Na concentration similar to that of plasma water – Crystalloid distributes throughout the extracellular space – Infusion of crystalloid will cause a fluid shift into or out of the intracellular space only if it creates an osmotic gradient between the extracellular and intracellular space – Isotonic saline does not change the osmolarity of the extracellular space – Therefore: Isotonic saline solution remains within and expands the extracellular space and has minimal effect on the intracellular space Maintenance Fluid and Electrolyte Requirements Maintenance: The replacement of normal ongoing losses – Normally serum Na concentration is approximately 140 meq/l and serum K concentration is approximately 4 meq/l – Maintenance solution replaces normal losses – Maintenance solution does not have an electrolyte concentration equal to serum because the electrolyte composition of urine and stool is not equal to that of serum Maintenance fluids commonly provided as a 5% dextrose solution – Dextrose provides some energy and prevents hypoglycemia » Spares protein – Cannot meet patient’s nutritional requirements with 5% (or 10%) dextrose Maintenance Requirements are a Function of Caloric Requirements 0-10 kg: 100 kcal/kg 10-20 kg: 50 kcal/kg > 20kg: 20 kcal/kg Examples: – 8 kg: 8 kg X 100 kcal/kg = 800 kcal. – 12 kg: 10 kg X 100 kcal/kg + 2 kg X 50 kcal/kg = 1000 kcal + 100 kcal = 1100 kcal – 20 kg: 10 kg X 100 kcal/kg + 10 kg X 50 kcal/kg = 1000 kcal + 500 kcal = 1500 kcal – 25 kg: 10 kg X 100 kcal/kg + 10 kg X 50 kcal/kg + 5 kg X 20 kcal/kg = 1000 kcal + 500 kcal + 100 kcal = 1600 kcal Water and Electrolyte Requirements are Determined by Caloric Requirements Requirements per 100 kcal: – 100 ml water (provided as a 5% dextrose solution) – 2-4 meq Na – 2 meq K – 2 meq Cl Plasma: Anion is a balance of Cl and base (bicarbonate) – Maintenance solution: Can provide some anion as Cl and some as base (lactate, citrate, phosphate) or can provide all of it as Cl – But: Providing large volumes of fluid (e.g., in DKA or hypovolemic shock) with all of the anion as Cl will promote a hyperchloremic metabolic acidosis Standard Maintenance Solution D5W with 20-40 meq/l Na Cl and 20 meq/l KCl (or KAcetate or KPhosphate) will work well as a maintenance solution in most pediatric patients – Can use D5 0.2% (or D5 0.3%) NaCl with 20 meq/l KCl (or KAcetate or KPhosphate) as maintenance solution – Recent article advocated routine use of isotonic saline solution for pediatric maintenance solution Some disease states: Another solution might be appropriate – E.g.: Sickle cell anemia patients may have a relatively high Na requirement due to high urinary Na losses – 0.9% NaCl (without dextrose) in head trauma patients – K should be used with caution or omitted in patients with renal insufficiency Water and Electrolyte Requirements Based on Weight Water: – 0-10 kg: 100 ml/kg – 10-20 kg: 1000 ml plus 50 ml/kg – > 20 kg: 1500 ml plus 20 ml/kg Electrolytes: – Na: 2-3 meq/kg – K: 1-2 meq/kg Water requirement is the same as with the caloric-based system Electrolyte requirement is greater than with caloric-based system: Electrolyte requirement is a direct linear function of weight Routine Use of D5 0.45% NaCl as Maintenance Solution in Older Patients Calculate water and electrolyte requirements on a per 100 kcal basis: Relationship between water and electrolyte requirements is fixed and does not change as weight increases But: If the water requirement is calculated on a per 100 kcal basis and the Na requirement is calculated on a per kg basis, then as the patient’s weight increases the Na requirement will increase at a greater rate than the water requirement – Heavier children will require a maintenance solution with a higher Na concentration Why: Because the water requirement does not increase linearly as weight increases: As weight increases the water requirement as expressed on a per kg basis decreases Routine Use of D5 0.45% NaCl as Maintenance Solution (Continued) Consider Na concentration of maintenance solution if estimate Na requirement to be 3 meq/kg (not 2-4 meq/100 kcal) – 10 kg: 30 meq Na in 1000 ml: 30 meq /l: – 20 kg: 60 meq Na in 1500 ml: 40 meq/l – 40 kg: 120 meq Na in 1900 ml: 63 meq/l – 70 kg: 210 meq Na in 2500 ml: 84 meq/l This explains why commercially available maintenance solutions exist which are designed for children below and above a specific weight – Remember discussion about providing some anion as base: This explains why commercial solutions may contain some anion in the form of lactate or citrate Dehydration Dehydration Good working definition in pediatrics: Loss of body fluid, usually predominantly from the extracellular space, due to decreased intake and/or increased losses – Most common cause is probably acute gastroenteritis – Failure to replace fluids lost from ostomies and drains with an appropriate solution may cause significant electrolyte imbalance and dehydration Patients with apparently acceptable intake may develop significant fluid and electrolyte imbalances – E.g.: Infant with a ventricular drain will lose a significant amount of Na in the ventricular fluid » Such an infant may develop severe hyponatremia if exclusively fed human milk (low Na) Classify Dehydration as to Type Isonatremic dehydration: Serum Na between 130 meq/l and 150 meq/l Hyponatremic dehydration: Serum Na < 130 meq/l Hypernatremic dehydration: Serum Na > 150 meq/l Serum Na and osmolarity – Hypernatremic patients are always hyperosmolar – Isonatremic patients are not always isoosmolar » E.g.: Serum Na 140 meq/l and glucose 600 mg/dl – Hyponatremic patients are not always hypoosmolar » E.g.: Serum Na 129 meq/l and glucose 800 mg/dl – Note: Isonatremic or hypernatremic patient with normal glucose may be hyperosmolar due to mannitol Ongoing Abnormal Losses Maintenance solution is designed to replace ongoing normal losses Ongoing abnormal losses: Diarrhea, ostomy drainage, chest tube drainage, ventricular fluid drainage Possible to measure electrolytes in the fluid but is usually unnecessary – May be useful if there is a large volume of drainage accompanied by significant electrolyte imbalance Nasogasric drainage: 0.45% (or 0.9%) NaCl with 20-40 meq/l KCl Ileostomy drainage: 0.9% NaCl with 10-20 meq/l KCl or KAcetate Clinical Findings in Dehydration History: Refusal to feed, vomiting, diarrhea, decreased urine output – Increased risk: Children with defective thirst mechanism, DI, impaired access to water Physical: Sunken fontanel, decreased tears, decreased skin turgor, tachycardia, weak pulses, cool extremities – Hypotension is a late finding which occurs only after compensatory mechanisms have failed Laboratory: Metabolic acidosis, increased BUN, increased creatinine, increased urine specific gravity Classify Dehydration as to Severity Mild: Earliest signs of dehydration – 30-50 ml/kg deficit (3-5% dehydration) Moderate: Signs of dehydration more pronounced – 60-100 ml/kg deficit (6-10%) Severe: Impending or actual circulatory failure – 90-150 ml/kg deficit (9-15%) Smaller children (e.g., < 2 years old) use 5%-10%-15% dehydration In larger children (e.g., > 2 years old) use 3%-6%-9% rather than 5%-10%-15% to avoid providing excessive volumes of fluid Alternative approach: IV rate of one and a half maintenance for mild to moderate dehydration and twice maintenance for moderate to severe dehydration Severity (Continued) Can use weight change to estimate the volume of the deficit if the change is recent (i.e., over 24 hours) and you are confident that the weights are reliable – Recent weight loss implies predominantly a water loss Degree of dehydration is an estimate, not precise (analogous to a visual estimate of serum bilirubin) Initially underestimating the degree of dehydration is not harmful so long as any existing or impending circulatory failure is recognized and treated appropriately Initially overestimating the degree of dehydration is not harmful so long as the overestimate is recognized and the fluid regimen is appropriately adjusted Isonatremic Dehydration Traditional Management of Isonatremic Dehydration 24 hour repair: Provide the deficit and one day’s maintenance over a 24 hour period Give half the total in the first 8 hours – Volume of fluid given during an emergency phase (i.e., bolus) is included as part of the first 8 hour’s fluids The second half is given over the remaining 16 hours Emergency phase: One or more 20 ml/kg boluses of 0.9% NaCl in moderate to severe dehydration Repair solution: Maintenance and deficit requirements combined – In isonatremic dehydration can use D5 0.45% (or 0.3%) NaCl with 20 meq/l KCl (or KAcetate) Repair of Isonatremic Dehydration (Example) 21 kg patient with 10% dehydration Total 24 hour requirement: 3620 ml » Maintenance: 1520 ml » Deficit: 2100 ml – 1810 ml in first 8 hour and 1810 ml in next 16 hours Emergency phase: 2 isotonic saline boluses for a total of 40 ml/kg (840 ml) over 1 hour Repair solution: D5 0.45% NaCl with 20 meq/l KCl – 1810 ml – 840 ml: 970 ml over next 7 hours: 139 ml/hr – 1810 ml in next 16 hours: 113 ml/hr Repair Solution in Isonatremic Dehydration: Assumptions Deficit is primarily from the extracellular space Serum Na concentration unchanged: – Therefore the deficit must have Na concentration approximately equal to that of plasma water: 150 meq/l – Na concentration of plasma water is higher than that of serum because serum contains solids such as albumin which reduce the Na concentration Ignore component of the deficit which consists of intracellular fluid with a low Na and a high K concentration Ignore maintenance electrolyte requirements because they are relatively insignificant compared with the deficit electrolyte requirements – Some authorities include the maintenance electrolytes in their calculations Repair Solution in Isonatremic Dehydration (Continued) 10 kg patient with 5% dehydration: » Maintenance: 1000 ml water » Deficit: 500 ml water and 75 meq Na – 1500 ml water and 75 meq Na: 0.3 % NaCl 10 kg patient with 10% dehydration: » Maintenance: 1000 ml water » Deficit: 1000 ml water and 150 meq Na – 2000 ml water and 150 meq Na: 0.45% NaCl Remember: Na deficit exists and must be replaced in isonatremic dehydration even though serum Na is normal – Na deficit: Na component of the isotonic volume loss Repair Solution in Isonatremic Dehydration (Continued) D5 0.45% (or D5 0.3%) NaCl with 20 meq/l KCl or KAcetate works well as a repair solution The Na requirement is determined by the deficit The greater the deficit relative to the maintenance requirements, the greater the Na concentration needs to be – Moderate to severe dehydration: D5 0.45% NaCl preferred over D5 0.3 % NaCl Chronic dehydration associated with a significant intracellular loss: Some patients may develop hypokalemia and require 30-40 meq/l of K in the repair solution Actual Calculations: Modified Finberg Technique Example: 12 kg patient with 10% isonatremic dehydration – Maintenance volume: 1100 ml – Deficit volume: 1200 ml – Deficit Na: 1.2 liters X 150 meq/l = 180 meq Repair the dehydration: Give 2300 ml of water and 180 meq of Na over a 24 hour period Technique: Give half over first 8 hours and the remainder over the next 16 hours – Give 20 ml/kg isotonic saline bolus if have deficit > 10% Modified Finberg Technique (Continued) 12 kg patient with 10% dehydration: Require 2300 ml of water and 180 meq of Na over a 24 hour period Emergency phase: 20 ml/kg X 12 kg = 240 ml of 0.9% NaCl » 240 ml of water » 37 meq of Na Repair solution » Water: 2300 ml - 240 ml = 2060 ml » Na: 180 meq - 37 meq = 143 meq » 2060 ml of water with 143 meq of Na » 5% dextrose solution with 69 meq/l of Na Give 1150 ml over first 8 hours and 1150 ml over following 16 hours – 1150 ml – 240 ml (bolus) = 910 ml » 910 ml/8 hr = 113.8 ml/hr – 1150 ml/16 hr = 71.8 ml/hr Summarize: 12 kg patient with 10% Isonatremic Dehydration Emergency phase: 20 ml/kg of isotonic saline » 240 ml of 0.9% NaCl » Dextrose free fluid bolus: Correct hypoglycemia separately if necessary Repair solution: 5% dextrose solution with 69 meq/l of Na – D5 0.45% NaCl close enough (77 meq/l Na) » Repair solution should include 20-40 meq/l of K to meet K needs and to replace any intracellular deficit – First 8 hours: 2300 ml/2 = 1150 ml - 240 ml = 910 ml/8 hr = 114 ml/hr – Subsequent 18 hours: 1150 ml/16 hours = 72 ml/hr Alternative Approaches to the Repair of Isonatremic Dehydration Give maintenance evenly over 24 hours but give half the deficit over the first 8 hours and the rest of the deficit over the next 16 hours – Complicated: Either use different IV bags for the maintenance and deficit fluids or change the electrolyte composition of the repair solution after the first 8 hours Estimating relative contributions of the extracellular and intracellular fluid to the overall deficit » Extracellular fluid: Na concentration of 150 meq/l » Intracellular fluid: K concentration of 150 meq/l – Unnecessary if K is provided in repair solution and is increased if hypokalemia develops during the repair Alternative Approaches to the Repair of Isonatremic Dehydration (Continued) Give one or more 20 ml/kg boluses of isotonic saline as needed and then run D5 0.45% (or 0.3%) NaCl at 1.5 or 2 times maintenance – Commonly done, not an unreasonable approach – Remember: Na concentration of D5 0.45% and D5 0.3% NaCl is significantly greater than maintenance requirements – True maintenance solution contains inadequate Na to effectively correct significant isonatremic dehydration Rapid correction of the deficit over less than 24 hours – E.g.: Give 100 ml/kg of isotonic saline solution over 3-6 hours to replace the deficit » Provide maintenance separately or by the oral route – May avoid hospitalization or shorten hospital stay Correction of Abnormal Osmolarity So long as actual or impending circulatory failure is treated appropriately with isotonic saline solution, the kidney will usually compensate if the degree of the water or Na deficit is underestimated or overestimated – Excessive urine output: May be a protective measure in a patient who is being rehydrated at an excessive rate Rapid correction of abnormal osmolarity – Potentially harmful » Hyponatremia: Central pontine myelinolysis » Hypernatremia: Seizures » DKA: Cerebral edema – Kidney will not protect against this