Biomimetic Hydrogels for Diabetic Neuropathies
advertisement

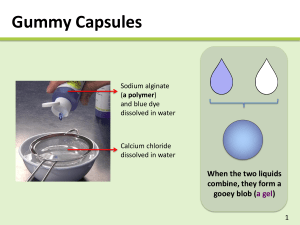
Natural Materials for Dural Replacement and Neuroprotection Vanessa Aguilar Project Plan October 27, 2010 Dural Replacement Therapy Needs Dura lesion complications: History of dural replacement • Meningitis • Cerebral spinal fluid leak • Pseudomeningocele • Arachnoiditis • Epidural abscess • 1895 first dural replacement1 • Mid 90’s xenograph and allograph were used • Since 70’s biosynthetic graft were investigated •14% of spinal surgeries requires a dural replacement technology2 http://www.meningitis-trust.ie/Meningitis.html http://www.nlm.nih.gov/medlineplus/enc y/imagepages/17146.htm Current dural replacement market •Gore-Tex (ePTFE) • Neuropatch (polyester urethane) • Duragen (Collagen) •DuraSeal (PEG-based spray) • Tisseel (Fibrin/trombin solutions) • Preclude (PTFE/ elastomeric Nasser, R. et al. Covidien http://www.emmgraphics.com/projects/covidien/spineseal/pdfs/09 fluoiropolymer) http://www.medcompare.com/details/16911/Duragen-Dural-GraftMatrix.html 1. Stendel et al. J Neurosurg, 2008. 2. Cammisa et al, Spine. 2000 _0924jallocase.pdf Anatomical Dural Overview Runza et al, Anesth Analg, 1999 http://members.cox.net/injections/images/esi_images/roots.jpg Stendel R et al.J Neurosurg 2008, Narotam P. et al, Spine 2004 Dural Replacement / Cranial Adhesion Barriers Barrier Device DuraGen (Integra Life Sciences) Synthecel Dura (Synthes) DuraSeal (Covidien) Adherus (HyperBranch) Description DuraGen/DuraGen Plus® is an innovative matrix designed to prevent peridural fibrosis and adhesions Cellulose – microbially grown cellulose PEG hydrogel Properties • Collagen based • Added cellulose layer for suturable performance Thick, very strong sheets of cellulose 100% synthetic CE approved for Water-tight sealant to be spinal applications applied during cranial or spinal surgeries for dura repair Advantages • FDA approved for neural applications • Natural-based material • Bioresorbable but degradation resistant FDA approved for dural replacement and wound dressing Phase III clinical trials FDA approved for cranial and spinal surgeries. Injectable and easy to use Spray-use, easy to use Disadvantages • Not easy to handle • Not an adhesion barrier • Attracts adhesions Very expensive Timely to grow Cannot be grown mass-scale • Set up required • Synthetic • Can be procoagulant • Nerve compression may ocur1 • Set up required • Synthetic • Can be procoagulant Model 1. Spotnitz, W and Burks, S. Transfusion. 2008 Synthetic surgical sealant – PEG hydrogel blend Plan of Work GOAL: To develop composite, dual-functioning materials that would serve to encourage healthy cell growth, wound healing and inhibits post-surgical scar tissue formation for neural applications. We aim to develop an all-in-one product to replace dural tissue as well as support healthy healing. AIM 1: Develop and characterize suturable anti-adhesion film / foam AIM 2: Develop bilayer biofunctionalized HAbased film AIM 3: Drug release studies • Biocompatible • Non-immunogenic • Non cell-adhesive / cytotoxic • Inhibits protein absorption • Mechanically robust • Watertight / sealing • Anti-fibrotic • Biocompatible • Bioabsorbable • Non-immunogenic • Dual functioning • Regenerative • Anti-adhesive • Mechanically robust • Cost effective • Clinically sized • Repositionable • Biocompatible • Effective at reducing adhesions • Encapsulate aspirin or ibuprofen • Tunable release rates Plan of Work GOAL: To develop composite, dual-functioning materials that would serve to encourage healthy cell growth, wound healing and inhibits post-surgical scarred tissue formation for neural applications. We aim to develop an all-in-one product to replace dural tissue as well as support healty healling. AIM 1: Develop and characterize suturable anti-adhesion film / foam AIM 2: Develop bilayer biofunctionalized HAbased film AIM 3: Drug release studies • Biocompatible • Non-immunogenic • Non cell-adhesive / cytotoxic • Inhibits protein absorption • Mechanically robust • Watertight / sealing • Anti-fibrotic • Biocompatible • Bioabsorbable • Non-immunogenic • Dual functioning • Regenerative • Anti-adhesive • Mechanically robust • Cost effective • Clinically sized • Repositionable • Biocompatible • Effective at reducing adhesions • Encapsulate aspirin or ibuprofen • Tunable release rates Material Properties Hyaluronic Acid Alginate Jeon et al, Biomaterials, 2009 http://www.madsci.org/posts/archives/apr2001/986571103.Bc.1.gif • Biocompatible • Bioabsorbable / non-immunogenic (nonanimal) • Very non-cell adhesive, polyanionic, hydrophilic • Antifibrotic1 (1% HMW HA) • Pro-angiogenic • Shown to reduce adhesion formation in animals and humans2 • Clinically used to reduce adhesions: Seprafilm, most effective and widely used anti-adhesion barrier on the market • Biocompatible • Low toxicity • Gels at physiological pH and temperature • Very non-cell adhesive, polyanionic, hydrophilic • Poorly immunogenic (depends on alginate purification)3 1. Massie et al, The Spine Journal,2005.. 2. Zawaneh et al, Tissue Eng Part B 2008. 3 Dusseault et al. Wiley InterScience, 2005 Anti Cell-Adhesion Properties 1. Well and film 2. Culture fibroblast cells 3. 1.5 hours in culture 4. Fix and stain for DAPI. 5. Validate cell-adhesion / non celladhesions www.biomedcentral.com Results Cell Adhesion Studies % Cell Adhesion 125 100 75 Alginate Alginate /GMHA 50 25 0 There is significant difference between control and films (p < 0.005) Alginate /GMHA /HA Control Cytotoxicity 1. Culture fibroblast cells 3. Place Alginate / HA film on cell medium 2. Seed cells in PLL coated TC coverslips 4. Wait 24 hours 5. Place Alginate / HA film supernatant on top of cells 4. Wait 24 hours 4. Stain coverslips with calcein / ethidium to label live / dead cells. 5. Evaluate cytotoxicity www.biomedcentral.com Results Cytotoxicity 140 % Cells 120 100 Control 80 Alginate 60 Alg-GMHA Alginate Alginate /GMHA 40 20 0 -20 Live Dead -40 There is no statistical difference between control and films in live and dead assay Control Antifibrotic studies (using laminectomy model) 1. Collect the tissue 2. Dehydrate in ethanol 3. Acid decalcify 4. Wait for 3 days 5. Slice every 50 um 6. Stain with H./E and Masson’s trichrome staining and analyze http://freepages.genealogy.rootsweb.ancestry.com/~gomery/gomorigeo.html Watertight Studies Manometer K. Hida et al. Surgical Neurology 65 (2006) 136–143 Protein Adsorption Studies Film Human serum albumen and human plasma fibronectin 1. Shake for 24 hours at 37˚C 2. Rinse with PBS 3. Addition of sodium dodecyl sulfate (SDS) 4. Stain with BCA protein assay reagent 5. Measure absorbance at 562 nm with UV/Vis spectrometer 6. Measure and analyze samples Huang and Yang, Polymers advanced technologies, 2009 Acknowledgments PI: Dr. Christine Schmidt, Graduate Students: Sarah Mayes Current Technologies • Autologous grafts • Pericranium or temporal fascia • Xenografts and allografts • Menengitis and Creutzfeldt-Jacobs Disease • Natural and syntethic materials • Gore-Tex (ePTFE) • Neuropatch (polyester urethane) • Duragen (Collagen) •DuraSeal (PEG-based spray) • Tisseel (Fibrin/trombin solutions) • Preclude ( PTFE/ elastomeric fluoiropolymer) http://www.medcompare.com/details/16911/Duragen-Dural-GraftMatrix.html http://medgadget.com/archives/2005/04/duraseal.html Stendel R et al. 2008, J Neurosurg 209:215-221 Results Cytotoxicity 140 % Cells 120 100 Control 80 Alginate 60 Alg-GMHA Alginate Alginate /GMHA 40 20 0 -20 Live Dead -40 There is no statistical difference between control and films in live and dead Alginate /GMHA/HA Control