blog ppt - WordPress.com
advertisement

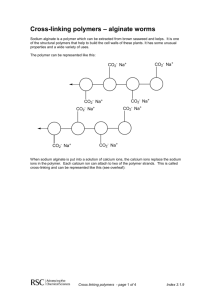
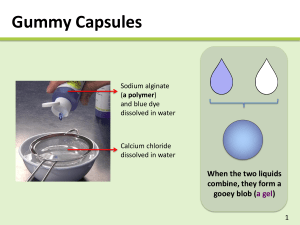
MOLECULAR GASTRONOMY AGENDA FOR TODAY • Introduction of myself and the plans for the period • Fill out pre-lesson quiz/survey • Interactive experiment: dark chocolate custard • Demo: liquid spheres • Interactive experiment: fizzy candy NOTES ABOUT LESSON • PLEASE be efficient today. I have lots of fun stuff planned, but only if we are fast • You’ll get to eat what you make! (And maybe a little of what I make!) • There’s going to be lots of science today. Don’t worry if you have a hard time understanding some of it. Feel free to ask questions! PRE-QUIZ/SURVEY • You have 2 minutes. GO! EXPERIMENT 1: GELIFICATION RELEVANCE? • How could we use gelification in other places in society? RELEVANCE? • Gluten-free pasta? Or flavored pasta? • Balistics gel? • Food in space? • Any other ideas? INGREDIENTS • 2g carrageenan • 300mL milk • 100g chocolate • 45g sugar PROCEDURE • Using hot plate, melt chocolate and sugar into milk • When all ingredients are completely mixed together, SLOWLY mix in carrageenan PROCEDURE • When mixture is almost at a boil, turn off hot plate • CAREFULLY pour mixture into each cup • Set aside the cups to cool. Try not to move them too much after that FINISHED PRODUCT! WHY DOES THIS HAPPEN? • Carrageenan is a polysaccharide • Polysaccharide definition: a number of sugar molecules bonded together. • Sugar molecules are polar • Polar definition: a compound bearing a partial positive charge on one side and a partial negative charge on the other WHY DOES THIS HAPPEN? WHY DOES THIS HAPPEN? • Water becomes semi-immobilized, forming a gel! • Carrageenan-specific traits: • Only dissolves when heated up • Gels at temperatures under 60° C DEMO: SPHERIFICATION RELEVANCE? • WD-50 faux egg • A server puts a fried egg on your table. You cut into the egg, and both yellow and white liquids flow out. It tastes like a pina colada! • WD-50, a NY molecular gastronomy restaurant, spherifies coconut and mango to look like a fried egg. A sweet surprise! RELEVANCE? • Tylenol Liqui-Gels? • Faux caviar? • Candy? • Any other ideas? INGREDIENTS • 1.5g sodium alginate • 1.5g calcium salt • 100g water • 100g iced tea PROCEDURE 1. Mix sodium alginate with iced tea, and water with the calcium salt, using the immersion blender 2. Let rest for ~30 minutes to let air bubbles escape 3. Using a pipette or syringe, drop the iced tea into the calcium bath slowly 4. Remove spheres from calcium bath, rinsing them in water 5. Consume soon after! PICTURES! WHY DOES THIS HAPPEN? • This is sodium alginate • Sodium ions make one bond, so the molecule is flexible and moves freely when dissolved in water WHY DOES THIS HAPPEN? • Iced tea & sodium alginate are dropped into a bath with calcium ions floating around • Calcium takes sodium’s place, but calcium needs two bonds to be happy! • So, it bonds with other molecules in the strand, making the long strand rigid WHY DOES THIS HAPPEN? • Rigid strand=not dissolved in water • Since the outside edge of the drop is first to bond with the calcium, it solidifies much quicker than the rest • Therefore, caviar! WHEN THIS DOESN’T HAPPEN • pH is too low: too many hydrogen ions, so they react with the alginate instead of calcium • Calcified liquid: adding the sodium alginate makes the liquid react before it can be made into the right shape, so it just becomes a big solid blob EXPERIMENT 2: EFFERVESCENCE RELEVANCE? • Where could we use this? RELEVANCE? • “Evaporated” soda? • Air supply? • Pop rocks? • Any other ideas? INGREDIENTS • 2g sodium bicarbonate (baking soda) • 2g citric acid • 10g powdered sugar PROCEDURE • Mix the three ingredients together well • Coat the candy in the powder • Eat! (Or wrap it for later) WHAT HAPPENS? • It may seem normal now • Just put it in your mouth, and it will begin to fizz! WHY DOES THIS HAPPEN? • Citric acid is an acid (well, duh) • Sodium bicarbonate is a base • Base definition: A chemical species that donates electrons/hydroxide ions, or accepts protons (basically, the opposite of an acid) WHY DOES THIS HAPPEN? WHY DOES THIS HAPPEN? • Reactions generally can’t occur when both reagents are solids • The powder must dissolve in your mouth to allow the reagents to move around and react with each other WHY DOES THIS HAPPEN? THANKS! • Homework • Due tomorrow • It’ll only take a couple of minutes, I promise! • If you have questions or want to learn more, email me! • I hope you had fun (and learned something)!