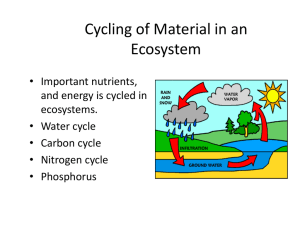
Cycling of P in freshwater systems PO 4 3
advertisement

Lecture Goals • To discuss why nitrogen and phosphorus are important nutrients in freshwater systems. • To trace how nitrogen and phosphorus move through freshwater systems, how they are transformed in the process. • To identify important ecological factors that influence movement and transformation of nitrogen and phosphorus. Why are N and P important? • N and P commonly the nutrients in greatest demand by plants and heterotrophic microbes relative to supply (i.e., limiting resources). • N commonly limiting in terrestrial systems, estuaries, and oceans. • P commonly limiting in freshwater systems. The problem with N • Nitrogen is an essential part of amino and nucleic acids • N is abundant on Earth (78% of atmosphere) • Only 2% available to organisms as reactive N (bonded to C, O, or H) • The rest is unreactive N (triple-bonded N2) The Nitrogen Cycle N2 Nitrogen Fixation N2 Cyanobacteria with Heterocysts Nitrogen Mineralization, Immobilization, and Uptake N2 Nitrification N2 Nitrification • Requires high O2 • Also very sensitive to pH → rates severely reduced at pH < 5.0 • When O2 or pH too low, then stops at intermediate forms: NO2- (nitrite) and N2O (nitrous oxide) • In freshwater systems, interested in nitrification because N needs to be in oxidized forms (NO3and NO2-) to partake in denitrification Nitrification at high pH Denitrification N2 Sites of Denitrification Debris Dams Emergent Plants Sediments • Lower metalimnion • Sewage treatment plants Who is doing the work and what are they working with? • N fixation: Cyanobacteria and terrestrial N-fixers Light + N2 • NH4+ immobilization and uptake: Microbes and plants NH4+, Light or No light, O2 or CO2 • Nitrification: Chemoautotrophic microbes NH4+, O2, moderate pH • Denitrification: Anaerobic bacteria and fungi NO3- (NO2- or N2O), Carbon, low O2 Nitrogen Distribution in Lakes Nitrogen in Rivers: Effects of surrounding forests Leaky Retentive Whole-Watershed Manipulations: Control vs. Cut and Leave WholeWatershed Results • Similar results from fire, but if build-up of charcoal in soil, then sorption of NO3-. • Can also have formation of NH4+ in atmosphere due to heat (energy from fire), then direct deposition. The 1998 Ice Storm Post-Storm N Spike Ice storm ~ Deforestation Ice storm ~ Deposition In-Stream Retention of N Nitrogen and Humans Nitrogen and Humans • Natural N-fixation: N2 → SOLAR ENERGY→ NH4+ • Industrial N-fixation via Haber-Bosh process N2(g) + 3H2(g) → HEAT → 2NH3(g) • Combustion of fossil fuels → NOx Nitrogen and Acid Rain H2SO4 HNO3 Delivery of N to Coastal Ecosystems Eutrophication of Coastal Ecosystems The Dead Zone The problem with P • P is a major cellular component, but occurs at VERY low levels in freshwater systems • P often limits primary production in freshwater systems Phosphorus in freshwater systems Phosphate PO43- Phosphorus in freshwater systems PO43- Organic P • Bound in living or decomposing material Phosphorus in freshwater systems PO43- Organic P Particulate P • Stuck to particles, especially metaloxides (e.g., FeOOH+) • Also in sedimenting organic particles • Carried to sediments Phosphorus in freshwater systems PO43- Organic P Dissolved P Particulate P • aka, SRP • Released via decomposition by anaerobic bacteria in sediment • Also some decomp. in water column Sources of P in freshwater systems • Runoff from land • Direct deposition from atmosphere • Pollution: wastewater, detergents, fertilizers, animal excretion Cycling of P in freshwater systems PO43- Biological Immobilization PO43- Cycling of P in freshwater systems PO43- Biological Immobilization Organic particles Sedimentation Cycling of P in freshwater systems PO43- Biological Immobilization Organic particles Sedimentation HOT SPOT Controls on P-exchange between sediment and water • Decomposition by anaerobic bacteria and turbulence at mud-water interface. Controls on P-exchange between sediment and water • Decomposition and turbulence at mudwater interface • Redox conditions within the sediment > Oxidized zones = retention by sorption > Anoxic zones = release by reduction Controls on P-exchange between sediment and water • Decomposition and turbulence at mudwater interface • Redox conditions within the sediment • Water acidity > As pH increases, PO43- released Phosphorus Distribution in Lakes Internal Loading of P • Change in “internal” conditions of lake cause massive release of P in sediments Mixing of sediment Increased pH Whole-lake anoxia Eutrophication of Lakes P in rivers • Pulse with high runoff or early stages of snowmelt • Generally see higher P levels in rivers and streams than in lakes because access to biota limited by flow dynamics The Nutrient Spiraling Model “How far downstream does the average atom of [YOUR FAVORITE NUTRIENT] travel before being taken up by the biota?” The Nutrient Spiraling Model P Labeled Nutrient 3(e.g., PO4 or NO3 ) + Estimating S Concentration Inert Tracer (e.g., Br or Cl) • S Low = Retentive • S High = Leaky Tracer Nutrient Downstream