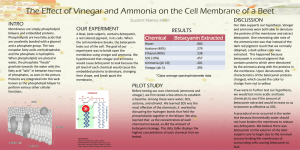
Sodium Hypochlorite & Ammonia Safety
advertisement

Sodium Hypochlorite First produced in 1789 in France by passing chlorine gas through a solution of sodium carbonate Today it can be produced in two ways: By dissolving salt in softened water, which results in a concentrated brine solution. The solution is electrolyzed and forms a sodium hypochlorite solution in water Using this process, hydrogen gas is also produced The second way is by adding chlorine gas to caustic soda, creating sodium hypochlorite, water, and salt Sodium Hypochlorite Clear/slightly yellowish in color Unstable Chlorine evaporates at a rate of 0.75 grams of active chlorine per day Disintegration occurs faster at warmer temperatures Should be kept in a climate-controlled environment Strong oxidizer Raises pH due to presence of caustic soda in hypochlorite Sodium Hypochlorite Uses As household bleach, hypochlorite is available as 3-8% An effective sanitizer as low as 2% As a disinfectant, hypochlorite is available as 10-15% 12% most common in water disinfection 15% most common in wastewater disinfection Large-scale usage in agriculture, and paint, lime, food, glass, paper, pharmaceutical, and waste disposal industries Can be used to prevent algae growth in cooling towers Sodium Hypochlorite Disinfection Used in place of chlorine gas As effective as chlorine gas Simple dosage Produces a measureable residual Can still create disinfection by-products Trihalomethanes & Haloacetic acids Sodium Hypochlorite Safety Various health effects can occur after exposure Inhalation: sore throat & coughing Skin: redness & pain Should use common sense when transferring from bulk tank to day tank Use respiratory and skin protection Ammonia Also referred to as aqueous ammonia, liquid ammonia, ammonia water, ammonium hydroxide One of the most commonly produced industrial chemicals in the US 80% of ammonia produced is used as fertilizer Also used in the treatment of water supplies, and in the manufacture of plastics, explosives, textiles, pesticides, and dyes Household ammonia cleaning solutions are between 5-10% ammonia Industrial ammonia solutions may be 25% or higher and are highly corrosive Ammonia At room temperature, ammonia is a colorless, highly irritating gas with a pungent, suffocating odor Corrosive Ammonia gas dissolves easily in water Is not highly flammable, but may explode when exposed to high heat Gas is lighter than air and will rise Exception to this is areas of high humidity – gas will form vapors that are heavier than air Ammonia in Water Treatment Used in conjunction with chlorine to create chloramines Commonly used when water travels a great distance Helps prevent the creation of disinfection by-products Ammonia Safety Has a greater tendency to penetrate and damage the eyes than does any other alkali Even low concentrations produce rapid eye irritation Repeated exposure can cause chronic cough, asthma, and lung fibrosis Odor provides adequate early warning, but ammonia also causes olfactory adaptation or fatigue, reducing awareness of prolonged exposure at low concentrations No antidote to ammonia poisoning Only supportive measures Ammonia Safety SCBA is recommended in response situations that involve exposure to potentially unsafe levels If contact with skin or eyes, immediate decontamination with copious amount of water is vital If inhaled, immediately remove that person to fresh air Ammonia should be stored/handled in a wellventilated area Wash hands immediately after handling Wear protective glasses and clothing