conductivity sensor calibration
advertisement

living with the lab
calibration of conductivity
sensors
living with the lab
DISCLAIMER & USAGE
The content of this presentation is for informational purposes only and is intended only for students
attending Louisiana Tech University.
The author of this information does not make any claims as to the validity or accuracy of the information
or methods presented.
Any procedures demonstrated here are potentially dangerous and could result in injury or damage.
Louisiana Tech University and the State of Louisiana, their officers, employees, agents or volunteers, are
not liable or responsible for any injuries, illness, damage or losses which may result from your using the
materials or ideas, or from your performing the experiments or procedures depicted in this presentation.
If you do not agree, then do not view this content.
The copyright label, the Louisiana Tech logo, and the “living with the lab” identifier should not be removed
from this presentation.
You may modify this work for your own purposes as long as attribution is clearly provided.
2
living with the lab
cal ∙ i ∙ brate [kal-uh-breyt]
-verb (used with object), -brat ∙ ed, -brat ∙ ing.
1. to determine, check, or rectify the graduation of (any
instrument giving quantitative measurements).
Calibration
•
•
•
Associate sensor output with salt concentration
Relate sensor output and salt concentration using
an equation (linear regression)
The goal is to be able to compute the salt
concentration based on sensor output
living with the lab
The Basic Idea
•
•
•
•
•
Adding salt to the water will increase the availability of Cl- ions at the anode
More ions at the anode will increase the rate at which chemical reactions can occur
The “electrical resistance” of the salt water will decrease as more salt is added to the water
The analog voltage on the + side of the 10kΩ resistor will increase as more salt is added
Correlating this voltage with the salt concentration will allow us to “calibrate” the conductivity sensor
5V
e-
e-
10 kΩ
anode – oxidation
cathode – reduction
(loss of electrons)
e-
(gain of electrons)
eCl-
ClCl2
Cl-
Na+
ClCl-
ClCl-
Na+
Na+
Cl
Cl
Na+
Na+
Cl-
Na+
Na+
Cl-
Na+
ion migration
OHee-
H
H2O
H
H2O
OH-
Cl-
Na+
Cl-
Cl-
Na+
Na+
Na+
Na+ is a spectator ion
living with the lab
The Circuit and Sketch
void setup() {
Serial.begin(9600);
// use a baud rate of 9600 bps
pinMode(1,OUTPUT);
// set pin1 as an output (pin1=TX)
Serial. write(12);
// clear screen & move to top left position
pin 3 = 5V when HIGH
Serial. write(128);
// move cursor to row 0, position 1
(set high periodically to measure conductivity)
Serial. write("Conductivity Sensor"); // print a text string starting at (0,1)
Serial. write(152);
// move cursor to row 1, position 4
Serial. write("Calibration");
// print a text string starting at (1,4)
Serial. write(189);
// move cursor to row 3, position 1
Serial. write("analog input=");
// print text string at (3,1)
Serial. write(22);
// turn cursor off to keep screen clean
pinMode(3, OUTPUT);
analog input 0
}
(measures voltage across 10kΩ resistor)
void loop() {
digitalWrite(3,HIGH);
delay(100);
int analogS=analogRead(0);
digitalWrite(3,LOW);
Serial.write(202);
Serial.print(analogS);
Serial.write(" ");
delay(1000);
}
// apply 5V to the conductivity sensor
// hold the voltage at pin 3 for 0.1s
// read voltage on + side of 10kohm resistor
// turn off power to the conductivity sensor
// move cursor to row 3, position 14
// print the analog input reading (0 to 1023)
// overwrite previously printed numbers
// delay 1 second between measurements
10 kΩ
living with the lab
Salt Concentrations
•
•
•
•
•
Each group of students should put about 1.5 inches of water in four bottles
The four bottles should contain . . .
• DI water
• 0.05% weight NaCl
• 0.10% weight NaCl
• 0.15% weight NaCl
Please take ONLY the amount that you will need to use TODAY
Be sure to label your water bottles
Swish a small amount of DI water around in your bottle to wash out
impurities before filling with calibration water
living with the lab
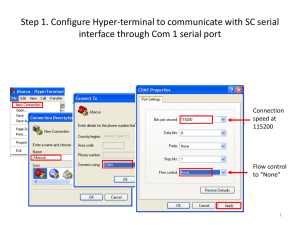
Steps
1. Configure your flow loop as required for homework
2. Implement the conductivity sensor circuit on your breadboard
3. Flush tank with DI water.
a. Pour enough DI water into your fishtank to fill the flow loop
b. Turn on the pump to run the flow loop for about a minute to “wash out” the impurities.
c. Prime the pump if necessary by switching the three-way valve to the drain
d. Turn the three-way valve toward the drain to flush the system
e. Repeat to completely clean the system
4. Fill the system with DI water
5. Collect calibration data for DI water
6. Fill the system with 0.05 wt% salt water. Flush once, and refill with 0.05 wt% salt water
7. Collect calibration data for 0.05wt% salt water
8. Repeat steps 6 and 7 for 0.10 wt% salt water and 0.15 wt% salt water
salt concentration
(%wt)
0.00
0.05
0.10
0.15
output to LCD
collect this data
living with the lab
Fit Output of Sensor to Salt Concentration
living with the lab
Determine Equation for Salinity Control
Swap dependent and independent variable when performing linear regression
salt concentration vs. output
0.16
• An equation like this will be used to
predict %wt salt based on sensor
output.
salt concentration (% wt)
0.14
0.12
0.10
y = 0.00004401e0.01512420x
R² = 0.99986136
0.08
• Try polynomial and power fits too to
see how they compare.
0.06
0.04
0.02
0.00
460
480
500
520
Output of Conductivity Sensor
540
560
• We will discuss the best form of the
equation to use later we write our
Arduino program to control salinity.
the end
living with the lab
The Circuit and Sketch (for old IDE)
If you haven’t downloaded the Arduino Integrated Development Environment (IDE) in a
while, then try program below.
void setup() {
Serial.begin(9600);
pinMode(1,OUTPUT);
Serial.print(12, BYTE);
Serial.print(128,BYTE);
Serial.print("Conductivity Sensor");
Serial.print(152,BYTE);
Serial.print("Calibration");
Serial.print(189,BYTE);
Serial.print("analog input=");
Serial.print(22,BYTE);
pinMode(3, OUTPUT);
}
// use a baud rate of 9600 bps
// set pin1 as an output (pin1=TX)
// clear screen & move to top left position
pin 3 = 5V when HIGH
// move cursor to row 0, position 1
(set high periodically to measure conductivity)
// print a text string starting at (0,1)
// move cursor to row 1, position 4
// print a text string starting at (1,4)
// move cursor to row 3, position 1
// print text string at (3,1)
// turn cursor off to keep screen clean
analog input 0
(measures voltage across 10kΩ resistor)
void loop() {
digitalWrite(3,HIGH);
delay(100);
int analogS=analogRead(0);
digitalWrite(3,LOW);
Serial.print(202,BYTE);
Serial.print(analogS);
Serial.print(" ");
delay(1000);
}
// apply 5V to the conductivity sensor
// hold the voltage at pin 3 for 0.1s
// read voltage on + side of 10kohm resistor
// turn off power to the conductivity sensor
// move cursor to row 3, position 14
// print the analog input reading (0 to 1023)
// overwrite previously printed numbers
// delay 1 second between measurements
10 kΩ