REACH *No Data, No Market*
advertisement

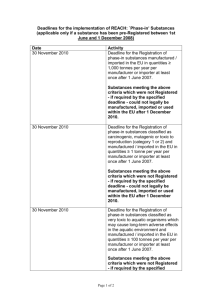
REACH ”NO DATA, NO MARKET” Elin Simonsson Lena Lundahl Anna Johansson ”NO DATA, NO MARKET” Art. 5 REACH; EC 1907/2006 OUTLINE Introduction Provisions Controversy Status/Discussion HISTORY BEHIND REGULATING INFORMATION Expensive and administratively heavy Internalizing cost of information Market failure Internal market – art. 114 TFEU MARKET-BASED REGULATION Compare with the EU ETS International aviation Global impact ART. 5 REACH […] substances on their own, in preparations or in articles, shall not be manufactured in the Community or placed on the market unless they have been registered […] DEFINITIONS Art. 3 REACH 3(1) Substance […] a chemical element and its compounds in the natural state or obtained by any manufacturing process […] 3(2) Article […] an object which during production is given a special shape, surface or design which determines its function to a greater degree than does its chemical composition […] OBLIGATION TO REGISTER Art. 6 REACH General obligation to register substances If the quantity exceeds 1 tonne annually, per manufacturer REGISTRATION Art. 7 REACH Criteria for registration of articles: Quantity exceeding 1 tonne per year and producer Substance in article is intended to be released under normal/reasonably foreseeable conditions of use REGISTRATION (CONT’D) Art. 10 REACH: Technical dossier Information about the manufacturer Identity of the substance Information on the manufacture and uses of the substance Classification and labeling Guidance of use Chemical safety report Study summary The tonnage range decides what information the registrant needs to submit (Annexes VII-XI) NOTIFICATION Art. 7 REACH Basic information about the substance might lead to an obligation to register Criteria for notification: Substance concentration in article exceeds 0.1%, and The substance has been classified as: Cancerogenic Mutagenic Toxic to reproduction Persistent, bioaccumulative and toxic, or Substances for which there is scientific evidence of probable serious effects to human health or to the environment JOINT SUBMISSION OF DATA Art. 11 REACH The lead registrant shall notify the Agency about: Classification and labeling Guidance on safe use Study summaries Proposals for testing Every registrant submits information specific to their own import/production Every single registrant responsible only for the information of their own manufacture or import SHARING OF COSTS Rules dispensed in different parts of the regulation Art. 27 REACH ”The previous registrant and potential registrant(s) shall make every effort to ensure that the costs of sharing the information are determined in a fair, transparent and non-discriminatory way.” CONTROVERSY 1. Privatization of information provision Obligation on private actors Explicit responsibility for the industry for the safety of its chemicals Internalization of costs CONTROVERSY (CONT’D) 2. Perverse incentives for the industry? Underestimation of risk Key governance problem of REACH Two principal mechanisms: Hierarchical control Peer review I. HIERARCHICAL CONTROL Dossier evaluation Examination of testing proposals Compliance check of registration II. DATA SHARING/JOINT REGISTRATION (PEER REVIEW) Title III, articles 25-30 of REACH REACH requires multiple registrants of the same substance to share data and to jointly submit their registration dossier Core principle to avoid unnecessary animal testing and reduce registration costs Mechanism differs depending on whether the chemical is a new (non phase-in) or an existing (phase-in) substance A. NON PHASE-IN SUBSTANCES: INQUIRY Duty to inquire whether a registration has already been submitted for that substance The Agency has to facilitate joint registration B. PHASE-IN SUBSTANCES: SIEF Duty to pre-register and to join a Substance Information Exchange Forum (SIEF) One joint submission for each substance Cost reduction Unnecessary animal testing avoided SIEFs have no prescribed legal form and are independently managed by the industry ECHA not involved CONTROVERSY (CONT’D) 3. Accountability 4. Risk assessment 5. Is it a workable regulatory strategy? Assessment of the quality of the data Risk assessment rather than hazard assessment Costs Do the costs to private actors exceed the public benefits? CONTROVERSY (CONT’D) 6. Practicalities Decisions need to be made about different issues: Risk identification Information quality Publication and sharing of information How is the information to be acted on? REACH REVIEW 2012 ”…special attention to the costs and administrative burden and other impacts on innovation.” DISCUSSION Is a focus on information a good way to regulate? From a market perspective? From an environmental perspective? Is it reasonable to put the obligation of information provision on the industry? Is it safe?