4.2 Guidelines for endorsement of additional manufacturing site
advertisement

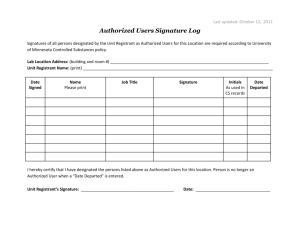
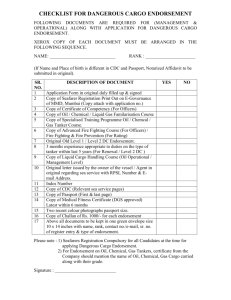
4.2 Guidelines for Endorsement of Additional Manufacturing Site (Approved in 325th meeting of RC held on 4th Jan., 2012) 1. The Committee decided that Toll Manufacturing and Additional Manufacturing Site are two different issues. The Committee recommends that as far Toll manufacturing is concerned, it should be regulated by the decision taken by the RC in its 296th meeting. However, endorsement of Additional manufacturing Site may be considered; 2. The Committee preliminarily recommends the following criteria/guidelines for accepting the requests for Additional Manufacturing Sites:(i) The site, proposed to be additional manufacturing site, should belong to the same registrant (it is not allowed on anyone else’s premises, whatsoever); (ii) the registrant should have registration for manufacture of the same insecticide/product in the country where the additional site is located; (iii) Letter of consent from already approved source and from new manufacturing site duly certified by the Designated National Authority (DNA), which shall be verifiable by the DNA of India; (iv) Additional declaration from already approved source/principals as well as from new manufacturing site with respect to the formulation being made by using technical same raw material & process from the source which is already approved by the RC in India; (v) all operations involved in the manufacturing of the insecticide for which the additional site is being considered should be fully operated by the same registrant and have the proof of manufacturing there by submitting an undertaking to this effect, duly certified by the DNA of that country; (vi) the registrant shall be responsible for manufacturing the product having identical chemical composition and specifications as that of the original registered source of the product; (vii) five batch analytical test report shall be submitted along with the application for the five batches manufactured at the source originally registered and the five batches manufactured at the proposed additional source; (viii) samples of all the five batch manufactured at the source originally registered and all the five batches manufactured at the proposed additional source shall be submitted along with the respective reference standards to the Sectt of CIB & RC for testing at CIL for verification; (ix) the samples and the reference standards should be valid at least for a period of six month from the date of delivery to the Sectt of CIB & RC; (x) documents supporting the claim of the registrant about the additional manufacturing site, duly certified by the Designated National Authority (like, ICAMA for China), shall be submitted along with the application, which shall in turn be verified by the DNA of India before considering the endorsement; (xi) intimation about the import of every consignment, proposed to be imported from the additional manufacturing site, shall be given to the Sectt of CIB along with the name of the port of entry into India and expected date of arrival as soon as the consignment is dispatched from the additional manufacturing site for arranging the testing of sample(s), if so considered necessary; (xii) Justification for approval of additional manufacturing site; and (xiii) failure to meeting any of the above mentioned criteria shall unconditionally empower the Registration Committee to reject application for such endorsement; or endorsement, if already granted. *****