C1b 5.3 Everyday emulsions
advertisement

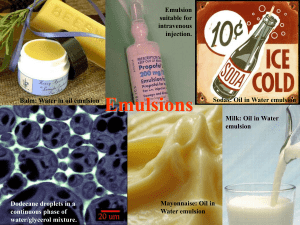
C1b 5.3 Everyday Emulsions What happens if you let ice cream melt and then re-freeze it? Separates into crunchy ice crystals and a buttery oily part Made from two runny liquids! Oil, water Egg yoke stops the oil and water from separating out Egg yoke is an emulsifier mayonnaise ice cream milk salad dressing What do these substances have in common? They are all…… OK – so what is an emulsion? Emulsions are foods made from a mixture of oil and water and they are important foods as they Oil are smooth and creamy. droplet Water But, oil and water don’t mix (immiscible) so we need a special substance which stops the water and oil separating out. We need an EMULSIFIER Emulsifiers stop oil and water from separating out, but how do they work? An emulsifier molecule _ This end is attracted to oil (Hydrophobic) This end is attracted to water (Hydrophilic) water oil droplet The charged oil droplets repel each other (like charges repel), keeping them spread throughout the water. _ _ _ _ _ _ _ _ _ The negative charges repel each other which stops the oil droplets from coming together and forming bigger drops. _ _ Observing an emulsion (milk) with a microscope Put a drop of one type of milk in the centre of the microscope slide. Place a cover slip at the edge, and support it with the mounted needle. Gently, lower onto the liquid in order to minimise air bubbles getting trapped underneath. Put the slide onto the microscope stage, and adjust for the lowest magnification and bring into focus. Repeat for other milk. Draw diagrams of what you see.