File
advertisement
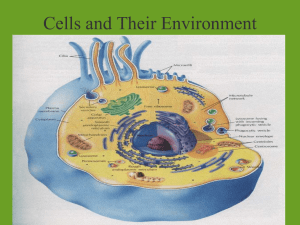
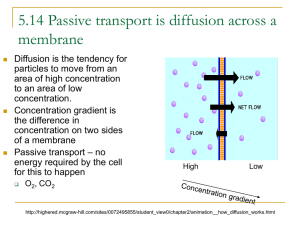
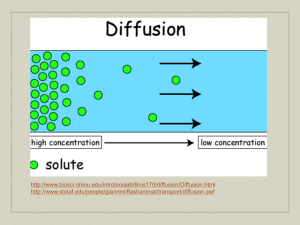
Cell membrane & Cellular Transport Biology Why do cells need cellular transport? Homeostasis (maintain stable internal environment for cell survival) Substances need to move in and out of the cell as they are needed Cell Membrane AKA Plasma Membrane Cell boundary that helps controls what enters the cell and what leaves the cell Permeable-being able to pass through Semi-permeable—some materials freely move through; some cannot Selective permeable-selects what may move in or out of the cell Cell Membrane Diagram Permeability-Which one is permeable, semi-permeable, not permeable at all? Passive Transport DOES NOT REQUIRE ENERGY Like riding a bike downhill; its easy, you coast, and not much energy is required Types of Passive Transport Diffusion Facilitated Diffusion Osmosis Diffusion Causes substances to move across the cell membrane (high concentration (a lot of particles to low concentration (few particles) Does NOT require energy Diffusion Demo Example: Perfume smell, Cookie smell Example: Making Kool-Aid http://highered.mcgrawhill.com/sites/0072495855/student_view0 /chapter2/animation__how_diffusion_wor ks.html http://www.youtube.com/watch?v=jinJNe Hvowo&feature=related Facilitated Diffusion Also called passive transport DOES NOT REQUIRE ENERGY Protein channels allow certain substances to pass through Example: HOV lanes in Atlanta http://highered.mcgrawhill.com/sites/0072495855/student_view0/chapt er2/animation__how_facilitated_diffusion_work s.html Osmosis Diffusion of water through a selectively permeable membrane DOES NOT REQUIRE ENERGY Active Transport REQUIRES ENERGY Movement of substances from lower concentration to higher Like you trying to move through crowded halls or ride a bike uphill; it takes a large amount of energy Types of Active Transport Endocytosis—Movement INTO the cell Exocytosis—Movement OUT of the cell Tonicity Remember, water flows from an area of high concentration to an area of low concentration through diffusion and osmosis Solution—mixture made of two substances mixed and evenly distributed Ex: Salt water Solvent—substance that does the dissolving Ex. Water Solute—substance being dissolved Ex. Salt Tonicity The solution outside the cell may have a different concentration of solute inside the cell Equilibrium-when water molecules are evenly distributed on both sides of the membrane Isotonic The concentration of solutes is the same on either side of the membrane Water enters and leaves the cell at the same rate. Diagram: Hypotonic Hypo-means LESS Less solute outside the cell; more water outside the cell Water pressure outside the cell pushes water into the cell Causes cells to swell or burst Animal cells may burst! Hypo—”Oh no! It’s going to burst!” Diagram: Hypertonic Hyper-mean MORE More solute molecules outside the cell; less water outside the cell Water pressure pushes water outside the cell CELL WILL SHRINK OR CONTRACT Plant cells can wilt Diagram: Three Types of Diffusion Types of Diffusion Review http://highered.mcgrawhill.com/sites/0072495855/student_view0 /chapter2/animation__how_osmosis_wor ks.html ADD TO NOTES BIO---means “life” ENDO—means “inside” EXO-– means “outside” LOGY—means “study of” CYTO---means “cell” OSIS– means “process or action”