Pharmacokinetics: Bioavailability
advertisement

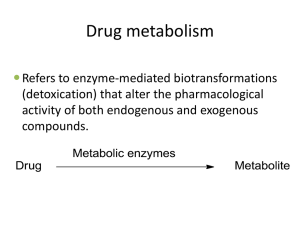
Pharmacokinetics: Bioavailability Asmah Nasser, M.D. Bioavailability The fraction of the administered dose reaching the systemic circulation in its chemically unchanged form. Labelled as “F” Bioavailability of a drug administered IV: 100% Bioavailability of a drug via other routes: ranges from 0% to 100% Bioavailability Destroyed in gut Oral Dose Not absorbed Destroyed by gut wall Destroyed by liver Drug dose at the systemic circulation! 3 How is bioavailability measured? To measure bioavailability, drug plasma levels are measured at different time points following various routes of administration Plasma levels are plotted against time Area under the curve (AUC) is measured If oral and iv doses are the same, then F = AUCoral/ AUCiv 4 Why are oral drugs less bioavailable? Because they undergo……. First pass metabolism Hepatic ‘First-pass’ MetabolisM Metabolism of drug by liver before drug reaches systemic circulation Drug absorbed into portal circulation, must pass through liver to reach systemic circulation Reduce the bioavailability of drug Orally administered drugs will have high FIRST PASS METABOLISM Parenteraly administered drugs will bypass the FIRST PASS METABOLISM to the major extent Ways to avoid First pass metabolism? Sublingual IV Rectal Intramuscular Bioequailance Two different formulations or two different brands (brand A and B) of a same drug give orally to the same person If they differ in bioavailibility and rate of absorption …both brand A and B are said to be Bioinequivalent…..this is common If they have same bioavailibility and rate of absorption …both brand A and B are said to be Bioequivalent…..this is uncommon Summary ◦ ◦ ◦ ◦ ◦ Bioavialability refers to The fraction of drug that reaches the systemic circulation as active metabolite The fraction of drug that reaches the systemic circulation as intact drug The fraction of drug that undergoes first pass metabolism The fraction of drug that eliminated by first pass metabolism The fraction of drug absorbed from the site of administration Summary Bioavailability First pass effect (metabolism) Bioequivalence Orally administered drugs will have high FIRST PASS METABOLISM Parenteraly administered drugs will bypass the FIRST PASS METABOLISM to the major extent 12 Biotransformation Asmah Nasser, M.D. Biotransformation 1. 2. ◦ ◦ It is a mechanism by which body: Terminates the action of the drug Sometimes leads to activation of drugs (pro-drugs) Most drugs are lipid soluble which means Favorable for absorption Slow removal from the body 3. Biotransformation hastens the excretion by making drugs less lipid soluble Types of metabolic reactions Phase I Phase II (addition of subgroups to –OH, NH2, -SH functions in the molecule) ◦ ◦ ◦ ◦ Oxidation Reduction Deamination Hydrolysis ◦ ◦ ◦ ◦ Glucuronide conjugation Acetylation Glutathione conjugation Glycine, methyl and sulfate conjugation Biotransformation reactions 1. Cytochrome P-450 mixed function oxidases (shortly called as CPY-450 enzymes) This is the largest enzyme responsible for biotransformation of drugs The CYP-450 superfamily is in turn subdivided into sub families CYP-1, 2, 3 etc. They are in turn again subdivided into CYP-1A, 3C etc. Factors affecting biotransformation Gender Genetic predisposition Co-existing pathological states Smoking Co administration of other drugs E.g. FPM of alcohol is lower in women than in men Smoking causes induction of enzymes and this increases the metabolism of drugs (e.g. theophylline) Co-administration of other drugs Based upon the fact that individual drugs can have on the drug metabolizing enzymes two things can happen: 1. Enzyme induction 2. Enzyme inhibition Enzyme Induction Some drugs induce (increase the levels) the drug metabolizing enzymes This will lead to increased rate of metabolism of other drugs This in turn will lead to decreased therapeutic effect of the second drug given E.g. rifampin is an anti TB drug, it is a known enzyme inducer (increase the drug metabolizing enzymes) Examples of drugs known to induce enzymes: carbamazepine, phenobarbital, phenytoin, rifampin Clinical application If oral contraceptives (OCP) are given to a lady who is on rifampin then the increase in the enzyme levels will lead to faster metabolism of the OCP and may lead to failure in contraception. Enzyme inhibition Some drugs can inhibit the drug metabolizing enzymes This can lead to reduced metabolism of other drugs This in-turn leads to increased action and/or toxic effects of the second drug E.g. cimetidine, an anti-ulcer drug is a potent inhibitor of CYP 3A4. Coadministration of warfarin leads to increased levels of warfarin and hence bleeding disorders List of Enzyme Inducers and Inhibitors Toxic metabolism Drug metabolism does NOT always lead to drug inactivation Some drugs can be converted to its active/toxic forms after metabolism The toxic substances thus produced can lead to severe injury of organs Acetaminophen metabolism Usually acetaminophen is conjugated to harmless glucuronide and sulfate metabolites In large doses Phase II metabolic pathways dominate and CYP-450 dependent system converts acetaminophen to a reactive metabolite (N-acetyl-p-benzoquinoneimine) Contd.. This reactive intermediate is conjugated with glutathione to a third harmless product In overdoses the glutathione store gets depleted, so the reactive intermediate combines with essential hepatic cell proteins This leads to cell death Rx: administration of sulf-hydryl donors (n-acetylcycteine) Note that ethanol intake induces the phase-I drug metabolizing enzymes and increases the formation of reactive intermediate of acetaminophen Summary Understand CYP-450 system Understand the co-administration of enzyme inducers and inhibitors and their effects on other drugs Understand the concept of a pro-drug Tylenol Toxicity, pathophysiology, treatment