AverageAtomicMass
advertisement

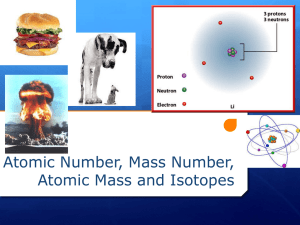
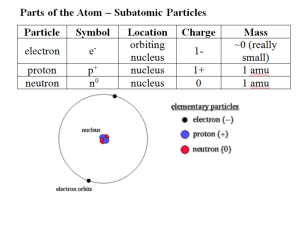
More about isotopes Atomic mass vs average atomic mass or atomic weight Atomic Mass The mass of a specific isotope of an element expressed in atomic mass units (amu) The atomic mass is the total mass of the protons, neutrons and electrons in the atom Atomic mass = mass of an atom This is what we refer to in our isotope expression, eg. Average Atomic Mass (also called Atomic Weight) This is the number that you see in the periodic table. 1 Atomic Number H Symbol of element 1.008 Atomic Weight This is the weighted average of the all of the isotopes of Hydrogen What is a Weighted Average? The isotopes of an element do not occur with equal frequency or amounts The atomic weights in the periodic table = weighted averages For example, the relative abundances for the three carbon isotopes are: Tabulated atomic weight value doesn't match any actual atom, but is closer to the weight for the most common isotope We’ll follow the steps of how to calculate a weighted average next…. carbon-12 98.9% carbon-13 1.1%, carbon-14 <001%. The "average" mass for the atoms of an element is dictated by the most abundant or common isotope Calculating Weighted Average weighted average = ( decimal fraction A) mass A + ( decimal fraction B) mass B Carbon-12 atomic mass = 12 amu; abundance = 98.9% Carbon-13 atomic mass = 13 amu; abundance = 1.1% Carbon-14 atomic mass = 14 amu; abundance = 0.001% Atomic weight = (0.989 * 12) + (0.011 * 13) + (0.00001 * 14) = 12.011 6 C 12.011 Average Atomic Mass Worksheet Homework - I will post answers on website Monday. You will have to show ALL of your work.