II. Thermal Energy
advertisement

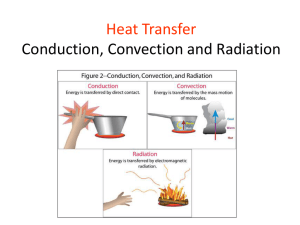
Vocabulary Thermal Energy – The SUM of all the molecular movement in a substance Temperature – The AVERAGE of the molecular movement in a substance Warmup Consider the free body diagram. If the sum of the tension forces is equal to the force of gravity, which description BEST applies? A) A book is at rest on a tabletop. B) A physics student rests a backpack upon one shoulder. C) A girl hangs by both hands, motionless, from a trapeze. D) A girl falls slowly to Earth while strapped to a parachute. Explanation A girl hangs by both hands, motionless, from a trapeze. If the forces are equal and opposite, there is no motion. There is not one but two tension forces work when a girl hangs by both hands, as illustrated by the two upward arrows in the free body diagram. Resting a backpack on your shoulder would be represented by only one tension force arrow. S8P2. Students will be familiar with the forms and transformations of energy. d. Describe how heat can be transferred through matter by the collisions of atoms (conduction) or through space (radiation). In a liquid or gas, currents will facilitate the transfer of heat (convection). S8P1. Students will examine the scientific view of the nature of matter. c. Describe the movement of particles in solids, liquids, gases, and plasmas states. Temperature The measure of the average kinetic energy (KE) of the particles in an object. Can be measured in degrees Celsius (°C), degrees Fahrenheit (°F), or kelvins (K). One kelvin is the same size as one Celsius degree. Temperature The lowest temperature on the Kelvin scale is 0 K, about -459 °F, which is called absolute zero: the temperature at which all molecular motion stops. It is not actually possible to reach absolute zero, although temperatures very close to 0 K have been reached in laboratories. Temperature Temperature As the temperature of an object increases, the average speed (KE) of the particles in random motion increases. The temperature of a substance does not depend on how much of it you have. The temperature of the tea in the cup is the same as the temperature of the tea in the teapot, even though there is more tea in the teapot. Thermal Energy The sum of the kinetic and potential energy of all the particles in an object ○ KE - movement of particles ○ PE - forces within or between particles due to position Depends on: ○ Temperature ○ Mass ○ Type of substance Thermal energy increases as temperature increases. Thermal Energy and Mass The more particles there are in a substance at a given temperature, the greater the thermal energy of the substance is. Although both soups are at the same temperature, there is more soup in the pot. So, the soup in the pot has more thermal energy than the soup in the bowl. Thermal Energy Which beaker of water has more thermal energy? B - same temperature, but more mass 80ºC A 80ºC B 200 mL 400 mL Thermal Expansion An increase in the size of a substance in response to an increase in the temperature of the substance. As temperature increases, particles move faster and spread out, leaving room for expansion. The opposite is known as contraction. Examples: expansion joints on bridges, bimetallic strips in thermostats, hot air balloon. Thermal Expansion Expansion Joints on Bridges The gaps in the bridge allow the concrete to expand (during warmer temperatures) and contract (during colder temperatures) without breaking. Thermal Expansion Bimetallic Strips in Thermostats A bimetallic strip is made of two different materials stacked in a thin strip. Because different materials expand at different rates, one of the metals expands more than the other when the strip gets hot, making the strip coil and uncoil. This opens and closes the circuit to make the heater turn off or on. Thermal Energy and States of Matter Matter exists in four states or phases: Solids Liquids Gases Plasmas Thermal Energy and States of Matter SOLID Tightly packed in a regular pattern; Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement; Vibrate, move about, and slide past each other GAS Well-separated with no regular arrangement; Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles Heat Thermal energy that flows from something at a higher temperature to something at a lower temperature Like work, heat is: measured in joules (J) a transfer of energy: ○ the amount of heat transferred depends on the difference in temperature between the objects. Heat Transfer Why does A feel hot and B feel cold? Heat flows from A to your hand = hot. Heat flows from your hand to B = cold. Heat flows until temperatures are equal (equilibrium). 80ºC A 10ºC B Warmup Conductor : A substance that transfers thermal energy easily Insulator : A substance the prevents or slows down the transfer of thermal energy Warmup – Unit 1 Review The Smith family is travelling in their car at 50 km/h due east. Mr. Smith is using cruise control to maintain a constant speed. Describe the net force acting on the Smith car. A)Net force equals zero. B)Net forces are unbalanced. C)Net force is positive and to the east. D)There is no way to determine net force. Explanation Net force equals zero. When an object travels at a constant speed in a straight line, the acceleration is zero. There is no change in speed or direction; that means no change in velocity. When an object is experiencing a net force of zero, it will continue to move at a constant velocity. Heat Transfer Thermal energy (heat) is transferred in three ways: Conduction – through matter by the collision of atoms (direct contact) Radiation – through matter or space by electromagnetic (EM) waves Convection – through liquids or gases by convection currents Conduction (Thermal Conduction) The transfer of thermal energy from one substance to another by collisions between the particles in matter (direct contact). Occurs most often in solids • Thermal energy is transferred when one end of a metal spoon is heated by a Bunsen burner. Conduction • When you heat a metal strip at one end, the heat travels to the other end. Conduction As you heat the metal, the particles vibrate. These vibrations make the adjacent particles vibrate, and so on and so on. The kinetic energy of these particles and the heat is passed along the metal. We call this conduction. Thermal Conductor Thermal Conductor – material that easily transfers heat Most metals are good conductors. Ex. Copper, silver, iron, steel, aluminum, glass In a piece of metal, there are electrons that are not bound to individual atoms, but can move easily through the metal. Collisions between these electrons and other particles in the metal enable thermal energy to be transferred more quickly than in other materials. Thermal Insulator Thermal Insulator – material that reduces or prevents the transfer of heat Nonmetals, wood, some plastics, fiberglass, Styrofoam, paper, and air are good insulators Materials, such as metals, that are good conductors of heat are poor insulators. Thermal Insulator Gases, such as air, are usually much better insulators than solids or liquids. Some types of insulators contain many pockets of trapped air. Building insulation is usually made of some fluffy material, such as fiberglass, that contains pockets of trapped air. The insulation is packed into a building’s outer walls and attic, where it reduces the flow of heat between the building and the surrounding air. Thermal Conductor vs. Thermal Insulator Why does metal feel colder than wood, if they are both at the same temperature? Metal is a conductor, wood is an insulator. Metal conducts the heat away from your hands. Wood does not conduct the heat away from your hands as well as the metal, so the wood feels warmer than the metal. Convection Convection - the transfer of heat by circulation or movement of currents in a liquid or gas Convection occurs mostly in liquids and gases. “Heat rises” is another way to think about convection. Examples: Warmer water at the surface of a lake or swimming pool Wind currents Hot air balloon Lower floors of a building being cooler than the top floor Convection What happens to the particles in a liquid or a gas when you heat them? The particles spread out and become less dense. This effects fluid movement. A fluid is a liquid or a gas. Convection • Cooler, denser fluids sink through warmer, less dense fluids. • In effect, warmer liquids and gases rise up. • Cooler liquids and gases sink. Convection • As water is heated, it becomes less dense. The warmer water rises through the cooler water above it. • At the surface, the warm water cools and becomes more dense. The cooler water then sinks to the bottom and the cycle repeats. • Convection currents transfer heat from warmer to cooler parts of the fluid. • In a convection current, both conduction and convection transfer thermal energy. Convection Cools at the surface Cooler water sinks Convection Convection current Hot water rises Convection Radiation Radiation – the transfer of energy by electromagnetic waves through empty space or matter. An electromagnetic wave is a wave that can travel through empty space or matter and consists of changing electric and magnetic fields. Energy is transferred from the Sun to Earth through radiation even though no matter is present. Heat energy can travel through a vacuum through radiation. Examples: A camp fire A microwave oven A light bulb Radiation Radiation • Air close to the ground is warmed by conduction. • The warmer air then rises by convection. • However, since there are no particles between the Sun and the Earth, heat CANNOT travel by conduction or by convection. •In nature, the Sun warms the Earth through radiation. •Land warms faster than water. ? RADIATION Radiation When radiation strikes a material, some of the energy is absorbed, some is reflected, and some may be transmitted through the material. The amount of energy absorbed, reflected, and transmitted depends on the type of material. Materials that are light-colored reflect more radiant energy, while dark-colored materials absorb more radiant energy. Radiation The transfer of energy by radiation is most important in gases. In a solid, liquid, or gas, radiant energy can travel through the space between molecules. Molecules can absorb this radiation and emit some of the energy they absorbed. Identify the Type of Heat Transfer That is Shown